Response of floret fertility and individual grain weight of wheat to high temperature stress: sensitive stages and thresholds for temperature and duration
P. V. Vara Prasad A B and Maduraimuthu Djanaguiraman AA Department of Agronomy, 2004 Throckmorton Plant Science Center, Kansas State University, Manhattan, KS 66506, USA.
B Corresponding author. Email: vara@ksu.edu
Functional Plant Biology 41(12) 1261-1269 https://doi.org/10.1071/FP14061
Submitted: 23 February 2014 Accepted: 31 May 2014 Published: 19 August 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Short episodes of high temperature (HT) stress during reproductive stages of crop development cause significant yield losses in wheat (Triticum aestivum L.). Wheat plants of cultivar Chinese Spring were grown at various temperature regimes at several stages of reproductive development for different durations. The objectives of this research were to (i) identify the stage(s) most sensitive to HT stress during reproductive development, and (ii) determine threshold temperature and duration of HT stress that decrease floret fertility and individual grain weight. Two periods (first at 8–6 days before anthesis and second at 2–0 days before anthesis) during reproductive development were most sensitive to short episodes (2 or 5 days) of HT stress, causing maximum decreases in floret fertility. Short episodes (5 days) of mean daily temperatures >24°C imposed at start of heading quadratically decreased floret fertility, with the values reaching close to 0% around mean daily temperature of 35°C; and floret fertility and individual grain weight decreased linearly with increasing duration (in the range from 2 to 30 days) of HT stress when imposed at start of heading or start of grain filling respectively. HT stress caused morphological abnormalities in pollen, stigma and style. The combination of lower floret fertility (leading to decreased grain numbers) and decreased individual grain weights can cause significant decreases in grain yield. Further research to search for genetic variability for these traits and use them in breeding programs to develop tolerant genotypes that can provide yield stability under current and future climates is warranted.
Additional keywords: abiotic stress, pollen, sensitive stage, sporogenesis, threshold, Triticum aestivum.
Introduction
Wheat (Triticum aestivum L.) is one of the most important food grain crops in the world in terms of area harvested, production and nutrition. Wheat is grown under diverse environmental conditions. Most wheat-growing regions often experience short episodes of above-optimum temperatures during reproductive stages of development, leading to significant yield losses. Crops grown in future climates are projected to experience higher frequency of short episodes of temperature extremes (IPCC 2013). These temperature extremes can cause significant yield losses if they occur during sensitive stages of development for certain durations. It is important to accurately quantify temperature responses, determine threshold for duration and sensitive stages to estimate impact on yield components and the implications of climate change and climate variability on wheat production.
Lobell and Field (2007) reported that wheat yield decreased globally by ~5.4% per 1°C rise in mean minimum or maximum temperatures from 1991 to 2002. Tubiello et al. (2002) projected that climate change will significantly decrease wheat production in rainfed areas of the USA by 30–40%. More recently, simulation study from field data in Kansas showed that a 1°C increase in projected mean temperature during reproductive stages can decrease wheat grain yield by 21% (Barkley et al. 2013). Controlled environment studies showed a 3–5% decrease in wheat grain yield for every one-degree increase in average temperature above 15°C (Gibson and Paulsen 1999).
Wheat plants were more sensitive to high temperature (HT) stress during reproductive stages than in vegetative stages (Farooq et al. 2011). Seed set mainly depends on the functionality of male and female gametes (pollen and ovule respectively). Environmental conditions during floral development and anthesis can influence performance of gametes and seed-set percentage. Tashiro and Wardlaw (1990) showed that transferring plants from 21/16°C to HT of 36/31°C for intervals of 2 days in the period from head emergence to 10 days after anthesis resulted in grain sterility in wheat. Grain sterility was inducted by HT stress at 2–3 days before anthesis, and this response was enhanced considerably by high humidity. High temperature stress during anthesis decreased seed set in peanut (Arachis hypogaea L.; Prasad et al. 1999, 2001), sorghum (Sorghum bicolor L. Moench; Prasad et al. 2008a), rice (Oryza sativa L.; Jagadish et al. 2007), dry bean (Phaseolus vulgaris L.; Prasad et al. 2002) and soybean (Glycine max L. Merr.; Djanaguiraman et al. 2013a, 2013b), resulting in lower seed yield. High temperature stress during pre-anthesis (microsporogenesis) causes poor pollen viability and fewer pollen grains, resulting in lower seed set in rice and sorghum (Prasad et al. 2006a, 2008a). High temperature stress during anthesis causes poor anther dehiscence and pollen tube growth and hampers fertilisation, resulting in lower seed set in rice (Jagadish et al. 2007). In wheat, Saini and Aspinall (1982) observed that HT stress (30/20°C, day/night) for 3 days during microsporogenesis and anthesis caused decreases in grain number. Similarly, maximum temperature above 31°C for a 5 day period ending at anthesis decreased seed set, leading to lower number of seeds per spike in wheat (Wheeler et al. 1996). High temperature stress during reproductive development altered pollen morphology and resulted in an abnormal exine wall, degeneration of tapetum cells and membrane damage, leading to decreased seed set in soybean (Djanaguiraman et al. 2013a, 2013b) and sorghum (Djanaguiraman et al. 2014). Anatomical evidence explaining reasons for failure of pollen germination under HT stress in wheat are not clearly documented.
High temperature (40°C daytime maximum) during the early grain filling stage caused maximum decrease in individual grain weight; the negative effects became progressively less at later stages of grain filling (Stone and Nicolas 1995). Stone and Nicolas (1998) reported that a day of high temperature (40/21°C day/night) during grain filling decreased the individual grain weight by 14% compared with a control (21/16°C day/night). Decreases in individual grain weight due to HT increased linearly with increased duration of stress, such that after the first day of HT stress, each additional day decreased individual grain weight by 1.6% (Stone and Nicolas 1998). Similarly, Hays et al. (2007) showed that a day of HT (40/18°C day/night) at 10 days after anthesis caused a 10–30% reduction in individual grain weight of wheat. Decreases in individual grain weight generally result from shortening of grain filling duration (Stone and Nicolas 1995; Prasad et al. 2008b).
Although studies on wheat have shown that episodes of HT (>32°C daytime maximum temperature) decrease spike fertility and individual grain weight, the relative sensitivity of various reproductive growth stages (booting, heading, anthesis and grain filling), critical thresholds for temperatures, and duration of stress for a particular cultivar have not been thoroughly quantified. Such knowledge will improve our understanding of wheat response to HT stress and better quantify the impacts of climate variability on grain yield. The objectives of this research were to (i) identify the stage(s) most sensitive to HT stress during reproductive development, and (ii) determine threshold temperature and duration of HT stress that decrease floret fertility and individual grain weight.
Materials and methods
This research was conducted in controlled-environment facilities in the Department of Agronomy at Kansas State University, Manhattan, KS, USA. A series of experiments were conducted to quantify response of wheat plants to HT stress.
Plant husbandry and growth conditions
In this study, wheat (Triticum aestivum L.) cultivar ‘Chinese spring’ was selected because of its sensitivity to HT stress (Qin et al. 2008). This cultivar has been widely used in genomic research and its genome has been sequenced using shotgun approach (Brenchley et al. 2012). Seeds of cultivar Chinese Spring were sown at 4 cm depth in 1.8 L pots (pot diameter at the top and bottom was 21 and 16 cm, respectively, pot depth was 20 cm) containing commercial Sun Grow Metro Mix 200 potting soil (Hummert International, Topeka, KS, USA). After emergence, plants were thinned to three plants per pot and maintained until maturity. A systemic insecticide, Marathon 1% G (granules) (active ingredient Imidacloprid 1-((6-chloro-3-pyridinyl) methyl)-N-nitro-2-imidazolidinimine, Hummert International), was applied to each pot at 4 g pot–1. The medium was fertilised with Osmocote (Hummert International) at 5 g pot–1 (controlled release plant food, 14 : 14 : 14%, N : P2O5 : K2O respectively) before sowing. To avoid water stress, all pots were irrigated daily and kept in trays containing water ~2 cm deep from sowing to maturity.
The seedlings were grown in growth chambers (Conviron Model E15, Winnipeg, MB, Canada) for the entire duration. Each growth chamber was 136 cm wide, 246 cm long and 180 cm high. The pots were randomly arranged within each growth chamber. Plants in each growth chamber were moved randomly every 7–10 day during non-stress period and every 1–2 days during the stress period, to avoid positional effects within the chamber. Temperatures in growth chambers were maintained in a square wave fashion. In all temperature regimes, daytime maximum temperature was held for 8 h from 0900 to 1700 hours. Similarly, the night-time minimum temperature was held for 8 h from 2100 to 0500 hours. The transition period between the daytime maximum and night-time minimum temperatures was 4 h and vice versa. Such temperature often occurs during sensitive stages of crop development in semiarid and humid regions. Relative humidity (RH) in all growth chambers was set at 80%. The photoperiod was 16 h (from 0500 to 2100 hours). In all growth chambers, the canopy level photon flux density (400–700 nm) was ~600 μmol m–2 s–1 provided by cool white fluorescent lamps (Philips Lighting Co., Somerset, NJ, USA). Air temperature and RH were continuously monitored at 15 min intervals in all growth chambers throughout the experiment using HOBO data loggers (Onset Computer Corporation, Bourne, MA, USA).
Treatments and observations
Response to high temperature stress: sensitive stages
Wheat plants were grown under optimum temperature (OT, 25/15°C, daytime maximum and night-time minimum; 16-h photoperiod and 80% RH) from emergence until onset of booting (Feekes growth stage 10.0, 15 days before anthesis). Thereafter, a set of 10 pots was transferred from the OT to HT conditions (36/26°C day/night, 14 h photoperiod and 85% RH) at 5 day intervals from 15 days before anthesis to 30 days after anthesis (a total of 10 treatments). The duration of HT stress for each treatment was 5 days. After the stress period, each set of 10 pots corresponding to a different treatment was returned to OT, where it remained until final harvest. Control plants (10 pots) remained under OT from start of sowing to final harvest.
In each treatment, spikelets on the middle portion of spikes on the main tillers of each plant were tagged (~30 plants from 10 pots) with cotton thread. On each spikelet, central or secondary florets (~80–100 florets) were marked with permanent ink marker and used to determine floret fertility. At maturity, the tagged florets were hand-harvested and dried at 40°C for 7 days. Individual tagged florets were checked for grain by pressing the floret between the thumb and the index finger. Both partially and fully filled tagged florets were used to determine floret fertility. Floret fertility percentage was estimated as the ratio of the total number of tagged florets to the number of grains from the tagged florets. The tagged florets were hand-threshed, counted and weighed. Individual grain weight was calculated by dividing the total grain weight by number of grains from the tagged florets.
To further pinpoint the exact stage with greatest sensitivity to HT stress, the experiment was repeated using similar protocols but shorter timing and duration of stress. Plants were grown at OT from emergence to start of transfer treatment. A set of 10 pots was transferred from OT to HT at 2 day intervals starting from ~14 days before anthesis until 4 days after anthesis (a total of 10 treatments). The duration of HT stress for each treatment was 2 days. After the stress period, each set of 10 pots corresponding to a different treatment was returned to OT, where it remained until final harvest. Control plants (10 pots) remained under OT from start of sowing to final harvest.
The procedure for tagging and determination of floret fertility and individual grain weight was similar to that mentioned above. In addition, at anthesis, florets were collected and pollen grains were extracted and spread on a microscopic slide to determine viability of pollen grains. Pollen viability was tested using a 2% triphenyl tetrazolium chloride stain. The stain forms an insoluble red formazan and stains live pollen a reddish purple. A drop of tetrazolium chloride was added to the dispersed pollen on microscopic slides. The number of pollen grains stained was recorded 30 min after staining by viewing them under a light microscope at ×10 magnification (Olympus BX 51, Center Valley, PA, USA). The percentage of viable pollen was estimated by dividing reddish purple-coloured pollen grains by the total number of pollen grains and multiplying by 100 (Djanaguiraman et al. 2014).
Response to high temperature stress: threshold temperature
To determine threshold temperatures at the start of heading, wheat plants were grown under OT (25/15°C, daytime maximum/night-time minimum; 14 h photoperiod and 80% RH) from emergence until onset of heading (Feekes growth stage 10.5). Thereafter, a set of 10 pots was transferred from OT to seven different temperature treatments (24/14, 27/17, 30/20, 33/23, 35/25, 38/28 and 40/30°C, day/night, giving daily mean temperatures of 19, 22, 25, 27, 30, 33 and 35°C) for a duration of 5 days. After the stress period, each set of 10 pots corresponding to different treatments was returned to OT, where they stayed until final harvest. Control plants (10 pots) remained under OT from the start of sowing through final harvest. The procedure of tagging and determination of floret fertility and individual grain weight was similar to those mentioned above.
Response to high temperature stress: threshold duration
To determine the threshold duration of HT at the start of heading and its effects on floret fertility, wheat plants were grown under OT (25/15°C, daytime maximum/night-time minimum; 14 h photoperiod and 80% RH) from emergence until onset of heading (Feekes growth stage 10.5). Thereafter, a set of 10 pots was transferred from OT to HT (35/25°C, day/night) for 11 different duration treatments (2, 4, 6, 8, 10, 12, 16, 20, 24 and 30 days). After the stress period, all 10 pots corresponding to each treatment were returned to OT, where they remained until final harvest. Control plants (10 pots) remained under OT from the start of sowing to final harvest. The procedures for tagging and determining floret fertility and individual grain weight were similar to those mentioned above.
To determine the threshold duration of HT at the start of grain filling and its effects on individual grain weight, wheat cultivar Chinese Spring was grown under OT (25/15°C; 14 h photoperiod and 85% RH) from emergence until onset of grain filling (Feekes growth stage 11). Thereafter, a set of 10 pots was transferred from OT to HT (35/25°C, day/night) for 11 different duration treatments (2, 4, 6, 8, 10, 12, 16, 20, 24 and 30 days). After the stress period, all 10 pots corresponding to each treatment were returned to OT, where they stayed until final harvest. Control plants (10 pots) of each treatment remained under OT from the start of sowing to final harvest. The procedures for tagging and determining floret fertility and individual grain weight were similar to those mentioned above.
Response to high temperature stress: anatomy and morphology of gametes and florets
To determine the impact of HT stress on morphology of gametes, samples were collected at anthesis from OT (25/15°C) and HT (35/25°C). At anthesis, the pollen grains were collected from the tagged panicle and dusted on double-stick carbon tape affixed to a carbon stub and immediately dipped in super-cooled ethanol for 1–3 s and stored at −80°C until further analysis. Similarly, the ovary along with style and stigma were taken carefully from individual florets, placed on double-stick carbon tape and processed like the pollen grains. The carbon stub was placed in a vacuum desiccator for ~2 min to remove excess moisture from the stub at the time of analysis. The pollen grains, stigma, style and ovary were viewed under a scanning electron microscope (SEM; Nova NanoSEM 430, FEI, Hillsboro, OR, USA) using a vCD detector (low voltage high contrast detector). The SEM was operated in a vacuum, 5 kV, with a spot size of 4 and pressure of 89.3 Pa. The pollen grain images were taken at ×1500, ×3000 and ×6000 magnification. The ovary, style and stigma images were taken at ×50, ×400 and ×500 magnification.
Quality control of growth chambers
Mean daytime and night-time temperatures in the OT and HT treatments were ± 0.5°C of the target temperatures and RH was within ± 10%. Quality of the temperature control and chamber performance was previously published (Pradhan et al. 2012).
Data analyses
Data from all the different experiments were statistically analysed using PROC GLM in the SAS software (SAS Institute, Cary, NC, USA). The experimental design for each experiment was a randomised complete block. The temperature of each growth chamber was assigned randomly, and plants were replicated within the chamber. There were 10 replications (10 pots) for all measurements. Standard error was shown as an estimate of variability, and means of different variables were separated by l.s.d. at probability level of 0.05. The response of floret fertility and individual grain weight to various temperatures and durations was tested for linear or curvilinear relationship and tested for significance and the best fit was identified using regression analysis in SAS.
Results
Response to high temperature stress: sensitive stages
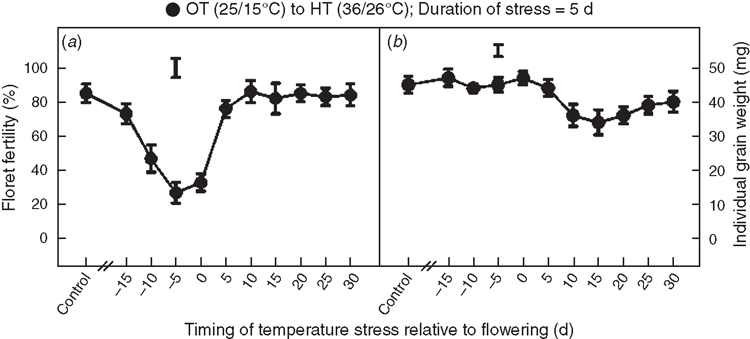
Compared with OT (25/15°C; mean daily temperature, 20°C), exposure to HT (36/26°C; mean daily temperature, 31°C) stress for 5 days significantly (P < 0.05) decreased floret fertility when imposed at 10, 5 or 0 days before anthesis (Fig. 1a). Maximum decrease in floret fertility occurred when stress was imposed at 5 or 0 days before anthesis. HT stress had no influence on floret fertility when stress was imposed 15 days before anthesis or at stages occurring at or beyond 5 days after anthesis (Fig. 1a). HT stress occurring at stages from 15 days before anthesis to 5 days after anthesis did not influence individual grain weight (Fig. 1b), but HT stress episodes occurring at stages from 10 and 30 days after anthesis significantly decreased individual grain weight to a similar extent (Fig. 1b).
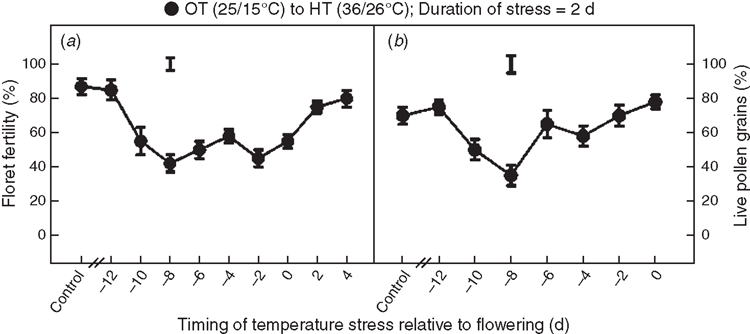
Exposure to short episodes (2 days) of HT stress (36/26°C) significantly decreased floret fertility when imposed anytime between 10 days before anthesis through 4 days after anthesis (Fig. 2a). Maximum decrease in floret fertility occurred when HT stress was imposed starting at 8 or 6 days before anthesis and at 2 or 0 days before anthesis (Fig. 2a). Similarly, HT stress decreased pollen viability when stress was imposed at 10 or 8 days before anthesis (Fig. 2b); however, the maximum decrease in pollen viability was recorded at 8 days before anthesis (Fig. 2b).
Response to high temperature stress: threshold temperature
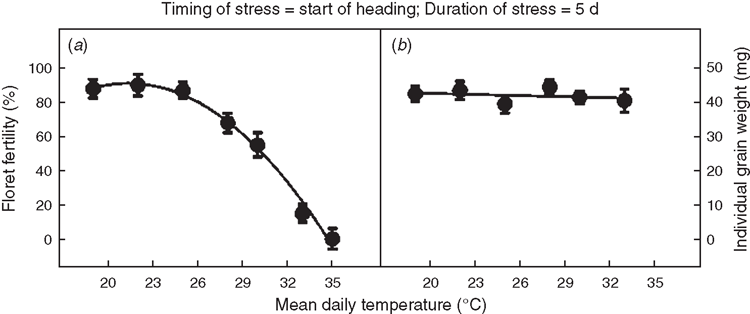
Floret fertility decreased significantly (P < 0.05) with increasing mean temperatures in the range of 24 to 35°C, when imposed for a duration of 5 days at start of heading. The response of floret fertility to temperature was best described with a quadratic function (Fig. 3a). Floret fertility decreased from ~85% at 24°C mean daily temperature to 0% at 35°C. Mean daily temperature in the specific range (19 through 35°C) when imposed at start of heading for a duration of 5 days had no significant effect on individual grain weight (Fig. 3b).
Response to high temperature stress: threshold duration
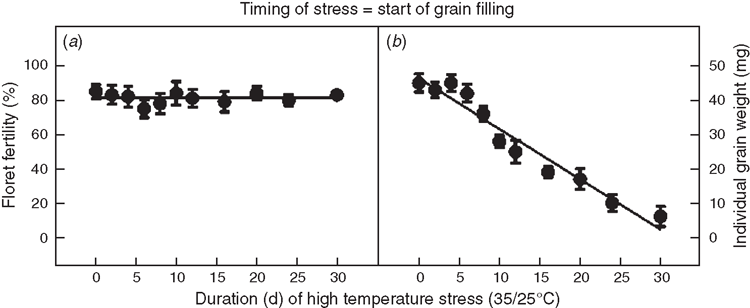
Plant exposure to HT stress (35/25°C; a mean daily temperature of 30°C) at the start of heading caused significant decreases in floret fertility (Fig. 4a). The response of floret fertility to duration was best described with a linear function across all data points (Fig. 4a). Individual grain weight was similar until 6 days of stress, and it decreased linearly thereafter with increasing duration (Fig. 4b).
Similarly, when HT stress (35/25°C, with mean daily temperature of 30°C) occurred at the start of rapid grain filling, it had no influence on floret fertility (Fig. 5a), which remained at ~80%. But increasing duration of stress significantly decreased individual grain weight. The response of individual grain weight to duration was best described with a linear function across all data points (Fig. 5b).
Response to high temperature stress: anatomy and morphology of gametes and florets
Exposure to HT stress (35/25°C) resulted in collapsed and desiccated pollen grains compared with OT (Fig. 6a, b, d, e). Pollen grains of OT plants had exine wall with smooth ornamentation in all directions, but pollen grains from HT plants had irregular surface patterns of more than 1 micron, as revealed by deeply pitted and non-smooth surfaces (Fig. 6c, f). In OT, the columellae heads of the exine were round, and in HT they were lost and non-uniform (Fig. 6c, f). The stigmas of HT-stressed plants were desiccated (Fig. 6g, j). The numbers of pollen grains adhered to stigma were lower in plants that experienced HT stress (Fig. 6g, j). The style and ovary were desiccated and flaccid in plants under HT stress (Fig. 6h, k), whereas plants from OT displayed turgid, and mucilaginous styles and ovaries, and had greater number of pollen grains (Fig. 6i, j).
Discussion
The response of plants to HT stress depends on the timing and the severity and duration of stress. Stages of reproductive development, particularly during meiosis of gametes, are sensitive to many environmental stresses, including HT and drought. Studies on wheat showed that drought stress during meiosis decreased the percentage of grain set (Dorion et al. 1996; Lalonde et al. 1997; Saini 1997). In the present study, the most sensitive periods (maximum decreases in pollen viability and floret fertility) to HT stress were between 8 and 6 days before anthesis (Figs 1, 2), which coincides with meiosis and tetrad formation stage of microsporogenesis, and between 2 and 0 days before anthesis which coincides with anthesis and fertilisation. Most grain crops are sensitive to HT stress during two specific periods of reproductive development, the first occurring before anthesis during floral bud development that normally coincides with micro- or mega-sporogenesis, and the second at the time of anthesis (Prasad et al. 2001, 2008a). Stress during these periods causes decreased seed set, leading to a lower number of seeds. The temperature-sensitive period before anthesis in cowpea (Vigna unguiculata was from 7 to 9 days before anthesis (Ahmed et al. 1992), and in common bean (Phaseolus vulgaris it was from 10 to 12 days before anthesis (Gross and Kigel 1994). Similarly, the HT-sensitive period in peanut was ~4 days before anthesis (Prasad et al. 2001), and in sorghum it was ~10 days before anthesis (Prasad et al. 2008a).
The decrease in floret fertility because of HT stress during microsporogenesis is due to loss of pollen viability (Fig. 2). Similar results were observed in several grain crops (Prasad et al. 2008a; Djanaguiraman et al. 2014). In the present study, abnormal exines with deeply pitted and non-smooth surface regions were observed in plants under HT stress (Fig. 6e, f). Exine originates from the tapetal cells, and the altered exine ornamentation under HT stress is an indication of disruption to tapetal cells. Tapetal cells provide nourishment to the developing pollen, and early degeneration of tapetal cells under HT stress affects translocation of nutrients to the developing pollen grains, leading to loss of pollen viability (Hess and Hesse 1994). Saini and Aspinall (1982) showed that HT stress causes structural and functional abnormalities in reproductive organs, which leads to failure of fertilisation or premature abortion of seed.
The second most sensitive period to HT is anthesis to fertilisation. The main processes occurring during this period include dehiscence of anthers, pollination, pollen reception by stigma, pollen germination, pollen tube growth in the style, and fertilisation and embryo formation. The present study showed that HT stress during the period of 2 to 0 days before anthesis decreased floret fertility even when the pollen was viable (Fig. 2b). The decreased floret fertility may be due to poor pollen germination and decreased rate of pollen tube growth, leading to unsuccessful fertilisation. The stigma, style and ovary of HT-stressed plants were deformed and desiccated, whereas these structures were normal and had copious exudates in plants under OT (Fig. 6g–l). Wheat stigma is of the dry type (Bailing and Ruilin 1991), and lipids, carbohydrates and proteins of stigma form the adhesive matrix for the dry stigma (Swanson et al. 2004). In plants under HT stress in the present study, the number of pollen grains attached to the stigmatic surface and the exudates levels were lower (Fig. 6h, k). In rice, spikelet fertility was strongly related to the number of germinated pollen grains (Jagadish et al. 2007, 2010; Prasad et al. 2006a) and the number of pollen grains on the surface of stigma; under HT, it was lower, leading to decreased fertilisation. Apart from this, pollen tubes are guided by signals from female cells to target the embryo sac. Water and lipids in the female reproductive tissue (stigma, style and ovary) provide directional cues that establish polarity (Wolters-Arts et al. 1998). The desiccated style and ovary under HT stress may not provide clear directional clues, leading to disoriented growth of the pollen tube. In addition, morphological characters such as stigma hyperplasia and stamen hypoplasia can result in failure of pollination (contact of pollen with stigma) and the fertilisation process (Takeoka et al. 1991). Changes in composition and concentrations of carbohydrates (Jain et al. 2007), lipids and reactive oxygen species (Djanaguiraman et al. 2013a, 2013b, 2014) in pollen gains result in pollen sterility. Additional research is required to test these principles in wheat.
A short period (5 days) of HT stress imposed at the start of heading did not cause significant decreases in individual grain weights (Figs 3b, 4b). One would expect that decreased spikelet fertility (lower grain number) may be negatively correlated with grain weight due to compensation (availability of more assimilates to developing grains). Although we observed slight increase in grain weight, it was not significant and there was no correlation between decreased spikelet fertility and corresponding increase in grain weight. After fertilisation, the embryo is relatively more tolerant to HT stress than gametes (Prasad et al. 2001), and the start of rapid seed filling is generally delayed under HT stress (Prasad et al. 2003). When HT stress was imposed for durations greater than 5 days; however, individual grain weight decreased linearly (Fig. 4b). Similarly, when HT stress was imposed after fertilisation at the start of the grain filling period, there was a linear decrease in individual grain weights and the response was cumulative (Fig. 5b). Yang et al. (2002) reported ~50% decline in average grain weight of 30 synthetic wheat genotypes subjected to HT of 10°C higher than the ambient (20/15°C), when stress was imposed from 10 days after anthesis until maturity. Castro et al. (2007) observed smaller grains in 14 spring wheat genotypes when exposed to HT stress from 15 to 22 days after anthesis. These decreases in individual grain weights could be associated with a shortening of the cell enlargement and dry matter accumulation phase in the grain, which occurs from 16 to 37 days after anthesis (Wang and Gifford 1995). Final grain weight is determined by the rate and duration of grain filling. Our earlier research with wheat showed that HT stress did not have large impact on the rate of grain filling, but it significantly decreased the grain filling duration, leading to smaller individual grain weights (Prasad et al. 2008b). High temperature accelerates the rate of grain filling, but shortens grain filling duration (Dias and Lidon 2009). For every 1°C above the optimal growing temperature of 15−20°C, the duration of grain filling was estimated to be reduced by 2.8 days in wheat (Streck 2005). However, slight increase in grain filling rates were not sufficient to compensate the losses caused by shorter grain filling periods (Stone and Nicolas 1995; Prasad et al. 2008b). Similar results were observed with grain sorghum (Prasad et al. 2006b, 2008a). Decreases in both seed filling rate and seed filling duration were observed in peanut (Prasad et al. 2003). We acknowledge that these results are from controlled environments and the fixed diurnal duration (8 h) of HT stress was longer than those commonly observed in the field conditions. Under field conditions, the diurnal duration of the temperature stress may be less, but is often more acute. Although, the sensitive stages or periods to HT stress may not be different under field conditions, they may have different thresholds. Further studies are required to confirm the threshold temperatures and their respective duration under field conditions.
In summary, research results indicated that (i) there are two periods (first at 8–6 days before anthesis and second at 2–0 days before anthesis) during reproductive development that were most sensitive to short episodes (2 or 5 days) of HT stress, causing maximum decreases in floret fertility; and (ii) short episodes (5 days) of mean daily temperatures >24°C imposed at start of heading quadratically decreased floret fertility, with the values reaching close to 0% around mean daily temperature of 35°C; and (c) floret fertility and individual grain weight decreased linearly with increasing duration (in the range from 2 to 30 days) of HT stress when imposed at the start of heading or start of grain filling, respectively. The decreases in floret fertility under HT stress were due to loss of pollen fertility and abnormalities in pollen, stigma and style. The combination of lower floret fertility (leading to decreased grain numbers) and decreased individual grain weights can cause significant decreases in grain yield of wheat. Further research to search for genetic variability in these traits and use them in breeding programs to develop tolerant genotypes that can provide yield stability under current and future climates is warranted.
Acknowledgements
We thank the Kansas Wheat Alliance, Kansas Wheat Commission, Coordinated Agricultural Project Grant no. 2011–68002–30029 (Triticeae – CAP) from the USDA National Institute of Food and Agriculture and USAID Feed the Future Innovation Lab on Climate-Resilient Wheatfor financial support. We thank summer intern students of Crop Physiology Laboratory for their help in conducting these experiments. We thank Dr Dan Boyle of Division of Biology at Kansas State University for helping with microscopic imaging. Mention of trademarks or proprietary products does not constitute a guarantee or warranty of the product by Kansas State University and does not imply its approval to the exclusion of other products, which may also be suitable. This is contribution no. 14–279-J from the Kansas Agricultural Experiment Station.
References
Ahmed FE, Hall AE, DeMason DA (1992) Heat injury during floral development in cowpea (Vigna unguiculata, Fabaceae). American Journal of Botany 79, 784–791.| Heat injury during floral development in cowpea (Vigna unguiculata, Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Bailing L, Ruilin Y (1991) Structure and development of stigmatic branches and style and their relation to pollen tube growth in wheat. Acta Botanica Sinica 33, 712–717.
Barkley A, Tack J, Nalley LL, Bergtold J, Bowden R, Fritz A (2013) ‘The impact of climate, disease, and wheat breeding on wheat variety yields in Kansas, 1985–2011. Bulletin 665.’ (Kansas State University Agricultural Experiment Station and Cooperative Extension Service)
Brenchley R, Spannagl M, Pfeifer M, Barker GA, D’Amore R, Allen AM, McKenzie N, Kramer M, Kerhornou A, Bolser D, Kay S, Waite D, Trick M, Bancroft I, Gu Y, Hou NX, Luo MC, Sehgal S, Gill B, Kianian S, Anderson O, Kersey P, Dovrak J, McCombie WR, Hall A, Mayer KFX, Edwards K, Bevan MW, Hall N (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491, 705–710.
| Analysis of the bread wheat genome using whole-genome shotgun sequencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslCnu7nL&md5=1c8e71cbab490652f473b7c419513a80CAS | 23192148PubMed |
Castro M, Peterson CJ, Rizza MD, Dellavalle PD, Vazquez D, Ibanez V, Ross A (2007) Influence of heat stress on wheat grain characteristics and protein molecular weight distribution. In ‘Wheat production in stressed environment’. (Eds HT Buck, JE Nisi, N Salomon) pp. 365–371. (Springer: Dordrecht, The Netherlands)
Dias AS, Lidon FC (2009) Evaluation of grain filling rate and duration in bread and durum wheat, under heat stress after anthesis. Journal Agronomy & Crop Science 195, 137–147.
| Evaluation of grain filling rate and duration in bread and durum wheat, under heat stress after anthesis.Crossref | GoogleScholarGoogle Scholar |
Djanaguiraman M, Prasad PVV, Boyle DL, Schapaugh WT (2013a) Soybean pollen anatomy, viability and pod set under high temperature stress. Journal Agronomy & Crop Science 199, 171–177.
| Soybean pollen anatomy, viability and pod set under high temperature stress.Crossref | GoogleScholarGoogle Scholar |
Djanaguiraman M, Prasad PVV, Schapaugh WT (2013b) High day- or night-time temperature alters leaf assimilation, reproductive success, and phosphotidic acid of pollen grain in soybean (Glycine max (L.) Merr.). Crop Science 53, 1594–1604.
| High day- or night-time temperature alters leaf assimilation, reproductive success, and phosphotidic acid of pollen grain in soybean (Glycine max (L.) Merr.).Crossref | GoogleScholarGoogle Scholar |
Djanaguiraman M, Prasad PVV, Murugan M, Perumal M, Reddy UK (2014) Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress. Environmental and Experimental Botany 100, 43–54.
| Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXislKnur8%3D&md5=63f826e85a9e0b800020c6435197c17fCAS |
Dorion S, Lalonde S, Saini HS (1996) Induction of male sterility in wheat by meiotic-stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiology 111, 137–145.
Farooq M, Bramley H, Palta JA, Siddique KHM (2011) Heat stress in wheat during reproductive and grain-filling phases. Critical Reviews in Plant Sciences 30, 491–507.
| Heat stress in wheat during reproductive and grain-filling phases.Crossref | GoogleScholarGoogle Scholar |
Gibson LR, Paulsen GM (1999) Yield components of wheat grown under high temperature stress during reproductive growth. Crop Science 39, 1841–1846.
| Yield components of wheat grown under high temperature stress during reproductive growth.Crossref | GoogleScholarGoogle Scholar |
Gross Y, Kigel J (1994) Differential sensitivity of high temperature of stages in the reproductive development of common bean (Phaseolus vulgaris L.). Field Crops Research 36, 201–212.
| Differential sensitivity of high temperature of stages in the reproductive development of common bean (Phaseolus vulgaris L.).Crossref | GoogleScholarGoogle Scholar |
Hays DB, Mason RE, Do JH, Menz M, Reynolds M (2007) Expression quantitative trait loci mapping heat tolerance during reproductive development in wheat (Triticum aestivum L.) In ‘Wheat production in stressed environments’. (Eds HT Buck, JE Nisi, N Salomon) pp. 373–382. (Springer: Dordrecht, The Netherlands)
Hess M, Hesse M (1994) Ultrastructural observations on anther tapetum development of freeze-fixed Ledebouria socialis Roth (Hyacinthaceae). Planta 192, 421–430.
| Ultrastructural observations on anther tapetum development of freeze-fixed Ledebouria socialis Roth (Hyacinthaceae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhvVOhsro%3D&md5=f60b2bf5a36b570e40cd42cd8e57ad94CAS |
IPCC (2013) Summary for policymakers. In ‘Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds TF Stocker, D Qin, GK Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex, PM Midgley) pp. 1–28. (Cambridge University Press: Cambridge)
Jagadish SVK, Craufurd PQ, Wheeler TR (2007) High temperature stress and spikelet fertility in rice (Oryza sativa L.). Journal of Experimental Botany 58, 1627–1635.
| High temperature stress and spikelet fertility in rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsFeju7c%3D&md5=4e86891437742ab3e35a64ae9de41dd6CAS |
Jagadish SVK, Muthurajan R, Oane R, Wheeler TR, Heuer S, Bennett J, Craufurd PQ (2010) Physiological and proteomic approaches to dissect reproductive stage heat tolerance in rice (Oryza sativa L.). Journal of Experimental Botany 61, 143–156.
| Physiological and proteomic approaches to dissect reproductive stage heat tolerance in rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGgu7fJ&md5=1a65f57861bbbd822cd26610b4dd14a0CAS |
Jain M, Prasad PVV, Boote KJ, Allen LH, Chourey PS (2007) Effect of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench). Planta 227, 67–79.
| Effect of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlyku73E&md5=c9640ff21cdda430d706458c1405b900CAS | 17680267PubMed |
Lalonde S, Beebe DU, Saini HS (1997) Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic stage water deficit. Sexual Plant Reproduction 10, 40–48.
| Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic stage water deficit.Crossref | GoogleScholarGoogle Scholar |
Lobell DB, Field CB (2007) Global scale climate-crop yield relationships and the impact of recent warming. Environmental Research Letters 2, 014002
| Global scale climate-crop yield relationships and the impact of recent warming.Crossref | GoogleScholarGoogle Scholar |
Pradhan GP, Prasad PVV, Fritz AK, Kirkham MB, Gill BS (2012) High temperature tolerance in Aegilops species and its potential transfer to wheat. Crop Science 52, 292–304.
| High temperature tolerance in Aegilops species and its potential transfer to wheat.Crossref | GoogleScholarGoogle Scholar |
Prasad PVV, Craufurd PQ, Summerfield RJ (1999) Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress. Annals of Botany 84, 381–386.
| Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress.Crossref | GoogleScholarGoogle Scholar |
Prasad PVV, Craufurd PQ, Kakani VG, Wheeler TR, Boote KJ (2001) Influence of high temperature during pre- and post-anthesis stages of floral development on fruit-set and pollen germination in peanut. Australian Journal of Plant Physiology 28, 233–240.
Prasad PVV, Boote KJ, Allen LH, Thomas JMG (2002) Effects of elevated temperature and carbon dioxide on seed-set and yield of kidney bean (Phaseolus vulgaris L.). Global Change Biology 8, 710–721.
| Effects of elevated temperature and carbon dioxide on seed-set and yield of kidney bean (Phaseolus vulgaris L.).Crossref | GoogleScholarGoogle Scholar |
Prasad PVV, Boote KJ, Allen LH, Thomas JMG (2003) Super-optimal temperatures are detrimental to peanut (Arachis hypogaea L.) reproductive processes and yield under both ambient and elevated carbon dioxide. Global Change Biology 9, 1775–1787.
| Super-optimal temperatures are detrimental to peanut (Arachis hypogaea L.) reproductive processes and yield under both ambient and elevated carbon dioxide.Crossref | GoogleScholarGoogle Scholar |
Prasad PVV, Boote KJ, Allen LH, Sheehy JE (2006a) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Research 95, 398–411.
| Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress.Crossref | GoogleScholarGoogle Scholar |
Prasad PVV, Boote KJ, Allen LH (2006b) Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum (Sorghum bicolor L. Moench) are more severe at elevated carbon dioxide due to higher tissue temperatures. Agricultural and Forest Meteorology 139, 237–251.
| Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum (Sorghum bicolor L. Moench) are more severe at elevated carbon dioxide due to higher tissue temperatures.Crossref | GoogleScholarGoogle Scholar |
Prasad PVV, Pisipati SR, Mutava RN, Tuinstra MR (2008a) Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Science 48, 1911–1917.
| Sensitivity of grain sorghum to high temperature stress during reproductive development.Crossref | GoogleScholarGoogle Scholar |
Prasad PVV, Pisipati SR, Ristic Z, Bukovnik U, Fritz AK (2008b) Impact of night time temperature on physiology and growth of spring wheat. Crop Science 48, 2372–2380.
| Impact of night time temperature on physiology and growth of spring wheat.Crossref | GoogleScholarGoogle Scholar |
Qin D, Wu H, Peng H, Yao Y, Ni Z, Li Z, Zhou C, Sun Q (2008) Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat (Triticum aestivum L.) by using wheat genome array. BMC Genomics 9, 432
| Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat (Triticum aestivum L.) by using wheat genome array.Crossref | GoogleScholarGoogle Scholar | 18808683PubMed |
Saini HS (1997) Effects of water stress on male gametophyte development in plants. Sexual Plant Reproduction 10, 67–73.
| Effects of water stress on male gametophyte development in plants.Crossref | GoogleScholarGoogle Scholar |
Saini HS, Aspinall D (1982) Abnormal sporogenesis in wheat (Triticum aestivum L.) induced by short periods of high temperature. Annals of Botany 49, 835–846.
Stone PJ, Nicolas ME (1995) Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. I. Grain growth. Australian Journal of Plant Physiology 22, 927–934.
| Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. I. Grain growth.Crossref | GoogleScholarGoogle Scholar |
Stone PJ, Nicolas ME (1998) The effect of duration of heat stress during grain filling on two wheat varieties differing in heat tolerance: grain growth and fractional protein accumulation. Australian Journal of Plant Physiology 25, 13–20.
| The effect of duration of heat stress during grain filling on two wheat varieties differing in heat tolerance: grain growth and fractional protein accumulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisl2qtrc%3D&md5=0f3bfb27909ea0d49af61cbb7ca1ba61CAS |
Streck NA (2005) Climate change and agroecosystems: the effect of elevated atmospheric CO2 and temperature on crop growth, development and yield. Ciência Rural 35, 730–740.
| Climate change and agroecosystems: the effect of elevated atmospheric CO2 and temperature on crop growth, development and yield.Crossref | GoogleScholarGoogle Scholar |
Swanson R, Edlund AF, Preuss D (2004) Species specificity in pollen–pistil interactions. Annual Review of Genetics 38, 793–818.
| Species specificity in pollen–pistil interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltlyitg%3D%3D&md5=558e8f575c9d717af16883f6db62833eCAS | 15568994PubMed |
Takeoka Y, Hiroi K, Kitano H, Wada T (1991) Pistil hyperplasia in rice spikelets as affected by heat-stress. Sexual Plant Reproduction 4, 39–43.
| Pistil hyperplasia in rice spikelets as affected by heat-stress.Crossref | GoogleScholarGoogle Scholar |
Tashiro T, Wardlaw IF (1990) The response of high temperature shock and humidity changes prior to and during early stages of grain development in wheat. Australian Journal of Plant Physiology 17, 551–561.
| The response of high temperature shock and humidity changes prior to and during early stages of grain development in wheat.Crossref | GoogleScholarGoogle Scholar |
Tubiello FN, Rosenzweig C, Goldberg RA, Jagtap S, Jones JW (2002) Effects of climate change on US crop production: simulation results using two different GCM scenarios. Part I: Wheat, potato, maize, and citrus. Climate Research 20, 259–270.
| Effects of climate change on US crop production: simulation results using two different GCM scenarios. Part I: Wheat, potato, maize, and citrus.Crossref | GoogleScholarGoogle Scholar |
Wang YP, Gifford RM (1995) A model of wheat grain growth and its applications to different temperature and carbon dioxide levels. Australian Journal of Plant Physiology 22, 843–855.
| A model of wheat grain growth and its applications to different temperature and carbon dioxide levels.Crossref | GoogleScholarGoogle Scholar |
Wheeler TR, Batts GR, Ellis RH, Hadley P, Morison JIL (1996) Growth and yield of winter wheat (Triticum aestivum L.) crops in response to CO2 and temperature. Journal of Agricultural Science 127, 37–48.
| Growth and yield of winter wheat (Triticum aestivum L.) crops in response to CO2 and temperature.Crossref | GoogleScholarGoogle Scholar |
Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen tube growth. Nature 392, 818–821.
| Lipids are required for directional pollen tube growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXivF2nt7o%3D&md5=39e4b28c83e5c918e45cb90d228e1286CAS | 9572141PubMed |
Yang J, Sears RG, Gill BS, Paulsen GM (2002) Growth and senescence characteristics associated with tolerance of wheat alien amphiploids to high temperature under controlled conditions. Euphytica 126, 185–193.
| Growth and senescence characteristics associated with tolerance of wheat alien amphiploids to high temperature under controlled conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltlSqsrk%3D&md5=971f8689815abb3069e206fb8708a574CAS |