A locus for sodium exclusion (Nax1), a trait for salt tolerance, mapped in durum wheat
Megan P. Lindsay A , Evans S. Lagudah A , Ray A. Hare B and Rana Munns A CA CSIRO Plant Industry, GPO 1600, Canberra, ACT 2601, Australia.
B NSW Agriculture, RMB 944, Tamworth, NSW 2340, Australia.
C Corresponding author. Email: rana.munns@csiro.au
Functional Plant Biology 31(11) 1105-1114 https://doi.org/10.1071/FP04111
Submitted: 24 June 2004 Accepted: 17 September 2004 Published: 18 November 2004
Abstract
Salinity affects durum wheat [Triticum turgidum L. ssp. durum (Desf.)] more than it affects bread wheat (Triticum aestivum L.), and results in lower yield for durum wheat cultivars grown on salt-affected soils. A novel source of salt tolerance in the form of a sodium exclusion trait, identified previously in a screen of tetraploid wheat germplasm, was mapped using a QTL approach. The trait, measured as low Na+ concentration in the leaf blade, was mapped on a population derived from a cross between the low Na+ landrace and the cultivar Tamaroi. The use of AFLP, RFLP and microsatellite markers identified a locus, named Nax1 (Na exclusion), on chromosome 2AL, which accounted for approximately 38% of the phenotypic variation in the mapping population. Markers linked to the Nax1 locus also associated closely with low Na+ progeny in a genetically unrelated population. A microsatellite marker closely linked to the Nax1 locus was validated in genetically diverse backgrounds, and proven to be useful for marker-assisted selection in a durum wheat breeding program.
Keywords: microsatellite marker, salinity, QTL.
Introduction
Soil salinity causes significant reductions in plant productivity, and consequent economic losses associated with reduced grain quality and yield of agricultural crops (Pitman and Läuchli 2002). Over 6% of the world's land is affected by either salinity or sodicity (FAO 2004). A large proportion of the Australian wheat belt is at risk of salinisation due to rising water tables (NLWRA 2004) and a further and larger part has soils that are sodic, and underlain with subsoil salinity (Rengasamy 2002). This subsoil salinity is formed in semi-arid zones (with annual rainfall less than 450 mm), and is transient in nature as it moves in and out of the root zone according to soil wetting and drying cycles (Rengasamy 2002).
Cultivars of durum wheat are more salt sensitive than bread wheat (Gorham et al. 1990; Rawson et al. 1988), and may yield less when grown on saline soils (Francois et al. 1986; Maas and Grieve 1990). The usual high price of durum wheat on the international market can bring a better return to farmers than bread wheat and other crops, so, breeding new cultivars of durum wheat with improved salt tolerance can allow growers more options in dealing with subsoil salinity. Marker-assisted selection is potentially the most efficient approach to developing cultivars that can tolerate saline soils.
There are three avenues by which to introduce salt tolerance into durum wheat: traditional breeding techniques using physiologically-based phenotyping, marker-assisted selection, and through transformation of genes known to improve Na+ exclusion or tissue tolerance. To increase salt tolerance of crops in terms of yield increases and associated economic gains, there is great potential for the introduction of salt tolerance traits into durum wheat using marker-assisted selection (Munns et al. 2002). This approach has successfully been used to introduce various agronomic traits into cereals, and overcomes the problems associated with wheat transformation and market acceptance (Koorneef and Stam 2001). Plant breeding using marker-assisted selection has a proven track record of successfully incorporating a stable trait into the genome of the target species. However, marker development is dependent on accurate phenotyping, requiring a quantitative measure of a specific trait. An understanding of physiological mechanisms is needed to identify such a trait.
Salt tolerance in the Tritiaceae is associated with sodium exclusion (Shah et al. 1987; Gorham et al. 1990; Husain et al. 2003). Bread wheat (hexaploid) cultivars have very low rates of Na+ transport to the shoot, and maintain a high K+ / Na+ ratio in leaves (Shah et al. 1987; Gorham et al. 1987). This ‘enhanced K+ / Na+ discrimination’ trait confers salt tolerance (Dvořák et al. 1994). A locus for the trait, the Kna1 locus, has been mapped to a region on the distal third of chromosome 4DL (Dubcovsky et al. 1996). However, durum wheat (tetraploid) cultivars lack this trait. Hexaploid wheat has three genomes, A, B and D, but tetraploid wheat has only the A and B genomes. A homoeologue of the Kna1 locus has not yet been found on the A or B genomes of tetraploid wheat. Recently, a novel source of Na+ exclusion was identified in a durum landrace (Munns et al. 2000). The landrace had very low rates of Na+ accumulation in the leaf blade, as low as bread wheat cultivars, and maintained a high rate of K+ accumulation, with consequent high K+ / Na+ ratios. The low-Na+ durum landrace had a K+ / Na+ ratio of 17 whereas the durum cultivars Wollaroi, Tamaroi and Langdon had K+ / Na+ ratios of 1.5, 0.7 and 0.4 respectively (Munns et al. 2000). The bread wheat cultivars Janz and Machete had K+ / Na+ ratios of 10 and 8, respectively. We considered the possibility that the low-Na+ durum landrace carries a homoeologue of the Kna1 locus. The low-Na+ trait was shown to confer a significant yield advantage at moderate soil salinity (Husain et al. 2003), indicating that this novel germplasm provides the opportunity to improve the salt tolerance of cultivated durum wheat.
Methods for selection of Na+-excluding individuals in wheat breeding populations are time consuming and expensive. In our case, the method involves growing plants in pots using a sub-irrigation system to provide a gradual and uniform exposure to NaCl to the plant, and the harvesting of a given leaf for Na+ accumulation. Although this screening method is very reproducible, it is labour intensive and requires a controlled environment. It is not possible to screen in the field or with large numbers of individual lines. QTL mapping and marker-assisted selection is a technique that has many advantages over phenotypic screening as a selection tool. Marker-assisted selection is non-destructive and can provide information on the genotype of a single plant without exposing the plant to the stress. The technology is capable of handling large numbers of samples. Although developing a QTL map is laborious, the markers identified may prove to be sufficiently robust to use as the sole selection tool for a specific trait in a breeding program. PCR-based molecular markers have the potential to reduce the time, effort and expense often associated with physiological screening. In order to use marker-assisted selection in breeding programs, the markers must be closely linked to the trait, and work across different genetic backgrounds.
The efficiency of genetic mapping has improved greatly in recent years, with the advent of high-density wheat consensus maps incorporating microsatellite markers (Roder et al. 1998; Harker et al. 2001), RFLP markers (GrainGenes 2004) and population-specific polymorphic fragments identified by the AFLP technique (Vos et al. 1995). The approach has been widely used to successfully map agronomic traits in a variety of cereal species. This paper describes a study that used microsatellite, RFLP and AFLP markers to map a Na+ exclusion trait and develop a marker closely linked to the trait in tetraploid wheat.
Materials and methods
Plant material
The source of the ‘Na+ exclusion’ trait for durum wheat, Triticum turgidum L. ssp. durum (Desf.), was the low-Na+ landrace Line 149, identified in a screen of 54 T. turgidum accessions (Munns et al. 2000; there referred to as selection number 126 775b).
The mapping population was derived from the cross between Line 149 and the Australian durum wheat cultivar, Tamaroi, using 100 F2 phenotyped individuals. The F2:3 progeny were also phenotyped (n = 15) and the data used to confirm the single-plant data for the F2 phenotype.
Two other populations were developed to verify the linkage of the marker to the Na+ exclusion trait, using crosses of Line 149 to two other high Na+ parents with unrelated genetic backgrounds: the cultivar Wollaroi, and the very high Na+ landrace line141 (identified by Munns et al. 2000). Populations of 100 F2 individuals were developed, and the F2:3 progeny means (n = 15) were used to verify the phenotype of the F2 individuals.
Two additional populations were developed to test the usefulness of the markers for detecting the Na+ exclusion trait in backcrosses with genetically unrelated breeding lines. Lines used were the advanced breeding lines BL960273 and BL961111, the latter now released as the cultivar Bellaroi. Low-Na+ F2 individuals from the Tamaroi × Line 149 cross were backcrossed into Tamaroi. BC1F2 generation plants were then backcrossed into the breeding lines BL960237 and BL961111, which were self-fertilised and backcrossed a second time. BC3F2 generation plants were used to verify the usefulness of the marker.
Phenotyping
Plants were grown according to the method of Munns et al. (2000) in a glasshouse in gravel culture using an automatic sub-irrigation system. Pots were sub-irrigated with half-strength Hoagland solution and 150 mm NaCl, and phosphate was reduced to 50 µm (Munns and James 2003). At 6 d after seedling emergence, when leaf 2 was half-expanded, 25 mm NaCl salt solution was added to the irrigation solution twice daily over 3 d to make up the final concentration of 150 mm NaCl. CaCl2 was added to bring the Ca2+ concentration to 8 mm. Daily photosynthetically available radiation (PAR) averaged 20 mol m–2 d–1 over the experimental periods. Glasshouse air temperatures were maintained at 23°C (day) and 20°C (night), and root temperatures were maintained at 19°C (day) and 16°C (night).
Na+ accumulation in the blade of the third leaf, 10 d after emergence, was measured in all plants according to Munns et al. (2000). Parental lines were replicated ten times. Leaf material was harvested, washed in distilled water, dried at 70°C for 2 d, extracted in 500 mm HCl at 80°C for 1 h, and Na+ concentration was measured by atomic absorption spectrometry (Varian Spectra AA-300, Melbourne, Vic.).
Genotyping
Genomic DNA
Plants that were grown in salt tanks for phenotyping were transplanted into soil and allowed to grow for approximately 4 weeks before DNA extraction. Leaf material from all plants was harvested and DNA was extracted as described by Lagudah and Appels (1991). Deoxyribonucleic acid was extracted from material in five populations. (1) Tamaroi × Line 149 population: 100 F2 individuals, 60 F3 families comprising the 30 extremes for Na+ concentration (the 15 with highest Na+ in the F2 generation, the 15 with lowest Na+) and 30 F3 families representing the distribution range of Na in the remaining 70 lines. (2) Wollaroi × Line 149 population: 30 extreme F2 individuals. (3) Line 141 × Line 149 population: 30 extreme F2 individuals, and 30 extreme F3 families. (4) BL961111 backcross population: 25 BC3F2 random individuals. (5) BL960273 backcross population: 25 BC3F2 random individuals. DNA was pooled from the 15 individual F2 plants with the lowest or highest Na+ concentrations respectively. This constituted the material used for the bulked segregant analysis.
AFLP markers
AFLP analysis was performed according to Vos et al. (1995) with Pst1 and Mse1 restriction enzymes and adapted primers. The sequences of AFLP adapters and primers are listed in Table 1. The selective primer set (Mse+3 and Pst+3) contained 144 primer combinations. For pre-amplification, Mse1 and Pst1 digested genomic DNA was amplified with Mse+1 and Pst+1 primers to produce a secondary template. Mse+3 and Pst+3 primers were used to selectively amplify AFLP fragments. Pst+3 primers (50 ng) were labelled with 33P-ATP (10 mCi μL–1) using T4 polynucleotide kinase (10 U mL–1) and PNK buffer. Samples were incubated at 37°C for 1 h followed by 70°C for 10 min to inactivate the kinase. The PCR touchdown cycle was: 94°C / 30 s, 65°C / 30 s, 72°C / 1 min, followed by 12 cycles where the annealing temperature dropped to 57°C over 12 cycles, followed by 23 cycles with an annealing temperature of 57°C. Selective amplification PCR product (20 μL) and 10 μL of loading dye (98% formamide, 10 mm EDTA pH 8.0, containing bromophenol blue and xylene cyanol as tracking dyes) was denatured at 95°C for 5 min. Denatured sample (3 μL) was loaded onto 6% denaturing gels. At completion of the run, the gel was neutralised in glacial acetic acid / 20% methanol solution for 20 min, dried on a glass plate (65°C for 5 h) and exposed to film (Kodak, Biomax MR, Eastman Kodak Co., Rochester, NY). AFLPs linked to Na+ exclusion were identified by ‘bulked segregant analysis’, i.e. on the basis of bands being present in one parent and the bulked DNA from the 15 extreme individual F2 plants relating to that parent, and not in the other parent and the bulked DNA from the 15 extreme individuals relating to it.

|
Microsatellite markers
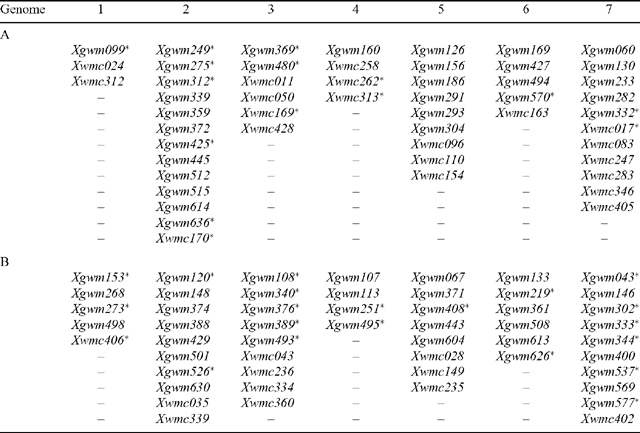
A group of 103 wheat microsatellite markers were used to screen the parental lines, Tamaroi and Line 149 (Table 2). Microsatellites were chosen on the basis of their map location in hexaploid wheat in an attempt to establish complete A- and B-genome coverage. The microsatellites that were polymorphic between the parents were used in mapping. All amplifications were performed in 20 μL aliquots containing 1.5 mm MgCl2, 2 μm primer pair, 200 μm dNTP, 200 μm 1× PCR buffer (Boehringer, Mannheim, Germany), 2 Units Taq DNA polymerase and 100 ng genomic DNA. Genomic DNA was amplified using a step-down PCR program: 95°C / 4 min, 15 cycles of 94°C / 30 s, 65°C–50°C / 30 s decreasing by 1°C per cycle, 72°C / 80 s, 30 cycles of 94°C / 15 s, 72°C / 45 s, followed by a 4°C holding step. The PCR products were separated using 1.8% Metaphor agarose gel. Microsatellites that produced polymorphisms between the parental lines were used to screen individuals in three different crosses. These were (i) 60 F2 individuals from the Tamaroi × Line 149 population (including the 30 extreme individuals and the 30 F2 individuals evenly distributed through the population), (ii) 30 extreme individuals from the Wollaroi × Line 149 population and (ii) 30 extreme individuals from the Line 141 × Line 149 population.
|
RFLP markers
Restriction endonuclease digestion and Southern hybridisation were performed according to the standard method (Sambrook et al. 1989). Parental lines were digested with restriction enzymes DraI, EcoRV, EcoRI, HindIII, NcoI, XbaI, BamHI, SacI, BglII, and NdeI, and screened with 20 RFLP markers for chromosome 2, based on published consensus linkage maps from hexaploid wheat. These markers (Xabc305, Xbcd402, Xcdo1376, Xpsr102, Xpsr107, Xpsr109, Xpsr112, Xpsr126, Xpsr131, Xpsr135, Xpsr137, Xpsr143, Xpsr146, Xpsr151, Xpsr609, Xpsr908, Xpsr932, XksuH11, XksuE16 and XksuD22) were selected according to preliminary microsatellite analysis that suggested a linkage to chromosome 2. The last two markers showed strong association with the Na+ exclusion trait in the 60 F2 individuals from the Tamaroi × Line 149 population. These RFLP markers were used to genotype the 30 extreme F2 individuals from the Wollaroi × Line 149 population, and 30 extreme individuals from the Line 141 × Line 149 population.
Mapping
A linkage map was created based on data arising from a phenotypic and genotypic screen of 60 F2 individuals from the Tamaroi × Line 149 population with 22 AFLP primer combinations, 36 microsatellites and 10 RFLPs. Single point linkage analysis (P<0.001) was performed using MapManager QTX version b13 software (Manly et al. 2001).
Results
Phenotyping and genetics of Na+ exclusion trait
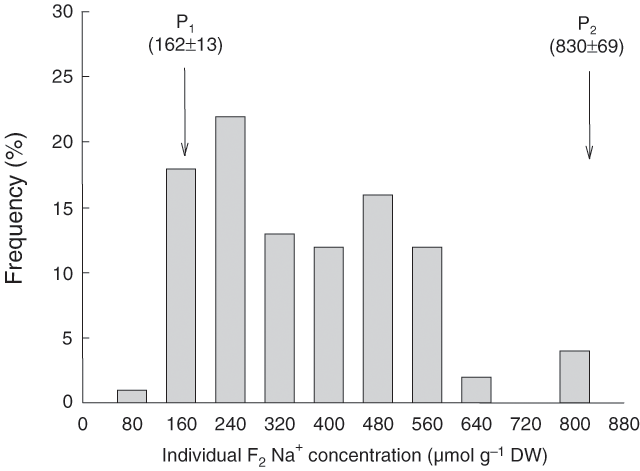
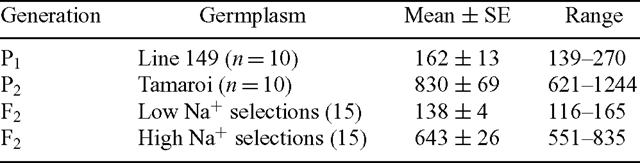
The low-Na+ parent (Line 149) had a 5-fold lower Na+ concentration than the high-Na+ parent (Tamaroi). The distribution of F2 individuals from the cross between them was skewed towards the low-Na+ parent, indicating that the low-Na+ trait was dominant (Fig. 1). Genetic analysis indicated that the progeny in the F2 generation segregated for Na+ accumulation in a 15 : 1 low : high Na+ ratio, indicating two genes of major effect, with interacting dominance action (Munns et al. 2003). Comparison between the F2 individuals and F3 progeny demonstrated that the trait has a very high heritability. The realised heritability was 0.90 (Munns et al. 2003). Differences between the parents and low / high-Na+ F2 individuals that were used in bulked segregant analysis are summarised in Table 3. Selective genotyping of the Tamaroi × Line 149 population was performed using bulked segregants based on pooled DNA from 15 low-Na+ F2 individuals and 15-high Na+. Molecular marker differences between the pair of pooled DNA constituted the basis for identification of putatively linked markers associated with the Na+ trait.
|
|
Genetic mapping
The first approach was an AFLP analysis of the parents, Tamaroi and Line 149, and the bulked segregants. Of the 144 primer combinations used, 22 combinations each produced at least five polymorphisms. Individual F2 progeny within the bulked segregants were then tested to validate the association of the putatively linked AFLP markers. One AFLP primer combination (AFLP 42) had a high association with the Na+ distribution.
A second approach was a QTL analysis based on 60 F2 individuals representing the full range of Na+ concentrations, and including all lines used in the bulked segregants.
This used the 22 AFLP markers identified to be polymorphic between the parents and the bulked segregants, and the microsatellites available for the A and B genomes. Of 103 microsatellite markers tested, 36 were polymorphic between the parents (Table 2).
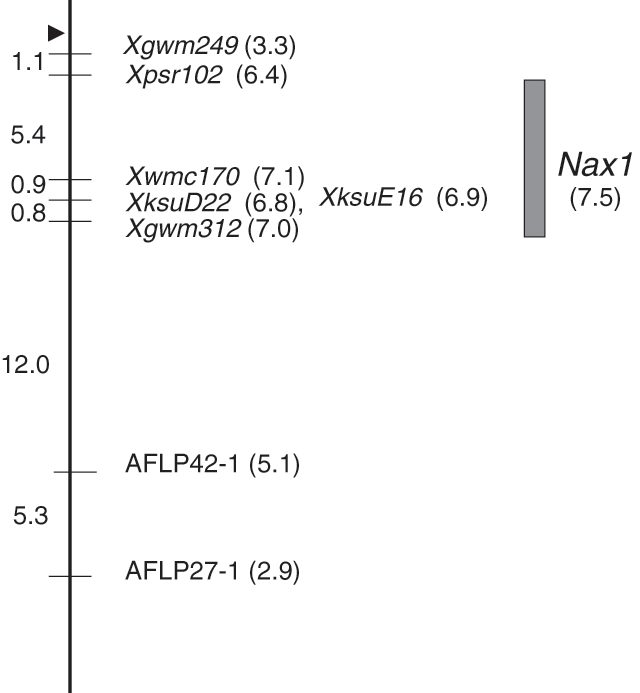
The genotypic data from the microsatellite and AFLP screen was imported into MapManager, and using a high stringency mapping approach (liklihood of odds, LOD, score of 3) several putative linkage groups were identified. Results from interval mapping indicated that a QTL with strong linkage to the Na+ exclusion trait was located on chromosome 2AL. The markers that map to this region are tightly linked (Fig. 2). In this initial screen, three microsatellite markers located on chromosome 2AL, Xgwm249, Xgwm312 and Xwmc170, were linked to the Na+ exclusion trait and had LOD scores of 3.3, 7.0 and 7.1, respectively. Six AFLP markers were linked to the microsatellite markers on chromosome 2AL. The two most tightly linked AFLP markers, AFLP42–1 and AFLP27–1, had LOD scores of 5.1 and 2.9, respectively.
|
In order to further increase the number of markers on the chromosome 2AL interval associated with the trait, RFLP markers that had previously been mapped to a similar interval in hexaploid wheat were selected. Of the 20 RFLPs from group 2 chromosomes tested, 10 were polymorphic between the parents and were tested on 60 F2 segregants. Three RFLP loci, namely Xpsr102, XksuE16 and XksuD22 were tightly linked to the Na+ exclusion trait and had LOD scores of 6.4, 6.9 and 6.8, respectively (Fig. 2).
Interval mapping of the Tamaroi × Line 149 population revealed that the QTL located on chromosome 2AL showed significant association with the Na+ exclusion trait having a LOD score of 7.5 (Fig. 2). This locus accounts for 38% of the phenotypic variation of the trait. The markers that had the closest association with the Na+ exclusion trait were the RFLP markers XksuD22 and XksuE16, and the microsatellites Xgwm312 and Xwmc170. The relative map locations of these four markers are consistent with previous reports of their location in the centromere region of chromosome 2AL. On the long arm of chromosome 2A in hexaploid wheat, Xpsr102 maps to a position 6.0 cM from the centromere, Xgwm312 maps 14.0 cM from the centromere, XksuE16 maps 15.5 cM from the centromere and XksuD22 maps 17.6 cM from the centromere (Roder et al. 1998; GrainGenes 2004). A specific genetic interval for the microsattelite Xwmc170 is not available. Based on the map location of the most closely linked markers, we predict that the Na+ exclusion locus, which we designate Nax1, is located on the long arm of chromosome 2AL, at an approximate position between 6 and 14 cM from the centromere (Fig. 2). The allelic contribution of the QTL located on chromosome 2A is predominantly from the Na+-excluding parent.
Marker validation
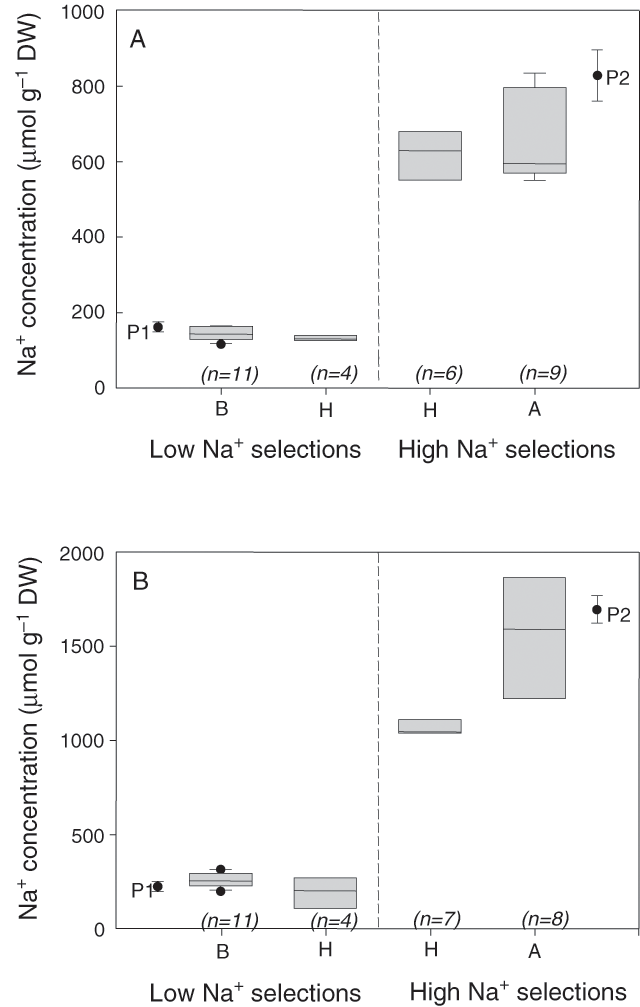
The two co-dominant microsatellite markers that were most closely linked to the Na+ exclusion locus on chromosome 2AL from the Tamaroi × Line 149 population were selected to validate whether alleles inherited from Line 149 were associated with the low Na+ exclusion trait. These markers were gwm312 (Fig. 3) and wmc170 (data not shown). The markers were first tested on the 30 extreme individuals from the Tamaroi × Line 149 population — the F2 individuals with the 15 highest and 15 lowest concentrations of leaf Na+. There was a strong relationship between the presence of the alleles inherited from the low Na+ parent, designated gwm312B (abbreviated as ‘B’), and the low Na+ uptake trait (Fig. 4A). Individuals with high Na+ uptake were associated with the alternative allele (abbreviated as ‘A’) from the high Na+ parent, Tamaroi. The markers also identified several heterozygous F2 individuals (‘H’) in both low and high Na+ uptake groups (Fig. 4A). The microsatellite wmc170 gave identical results as gwm312 (data not shown), because no recombinants between the markers were identified among the extreme subset of low and high Na+ uptake F2 individuals.
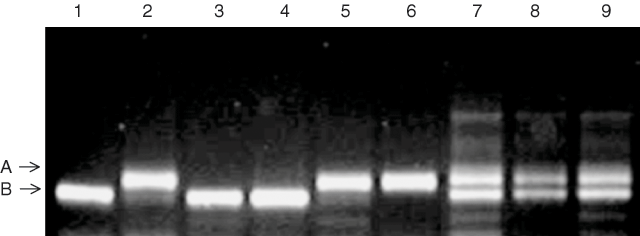
|
|
The markers were tested on a different population, resulting from a cross between Line 149 and a durum landrace with exceptionally high leaf Na+, Line 141. Tamaroi and Line 141 are genetically unrelated (Husain et al. 2003; Munns et al. 2003), therefore, this was a stringent test of the ability of the marker to identify individuals with the Na+ exclusion trait in different genetic backgrounds. The phenotype of each of the F2 individuals was determined using the standard phenotypic screen, and the 30 extreme F2 individuals were selected for marker validation (Fig. 4B). The results again showed a close linkage with the Na+ exclusion trait, in that the low Na+ individuals were homozygous B or heterozygous (H), and the high Na+ individuals were homozygous A or heterozygous (Fig. 4B). In this subset of extreme F2 individuals from the 141 × 149 population there were recombinants between the two microsatellites in that three of the low-Na+ individuals were homozygous B for gmw312 but heterozygous for wmc170, and that four of the high-Na+ individuals were homozygous A for gms312 but heterozygous for wmc170 or vice versa (data not shown). This suggests more extensive recombination at meiosis for the two landraces than for one landrace and a cultivar. However, in neither case were the low-Na+ individuals homozygous for the allele for the high-Na+ parent, or vice versa.
Validation for marker-assisted selection in a breeding program
To test the usefulness of the microsatellite markers in identifying the Na+ exclusion trait in a breeding program, three populations of different genetic background were selected and screened with the microsatellite marker gwm312. These populations resulted from an initial cross between Line 149 and Tamaroi, selection of the lowest Na+ F2 individuals, which were then crossed again with either Tamaroi or two other advanced durum breeding lines, selfed, and this process repeated two more times (see Materials and methods). These breeding lines had even higher leaf Na+ concentrations than did Tamaroi (Table 4). Then, 25 BC3F2 individuals from each population were assessed for Na+ exclusion using the standard phenotypic screen, and genotyped using gwm312. There was high correlation between low Na+ concentrations and the presence of the B allele of gwm312 in all three populations (Table 4). All the lowest Na+ individuals in each population were homozygous for the B allele, and all the highest Na+ individuals were homozygous for the A allele (Fig. 5). As was observed in the initial verification of the marker from the Tamaroi × Line 149 cross, several heterozygous individuals were identified in both the high- and low- Na+ classes (Fig. 5). However, the most salt-tolerant individuals carried only the B allele.
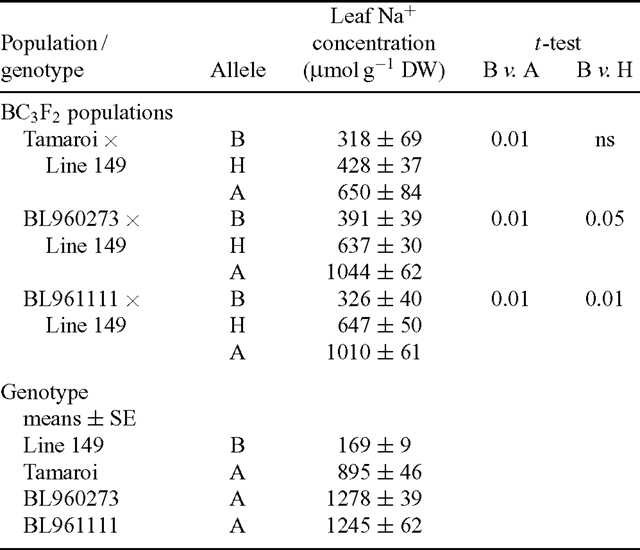
|
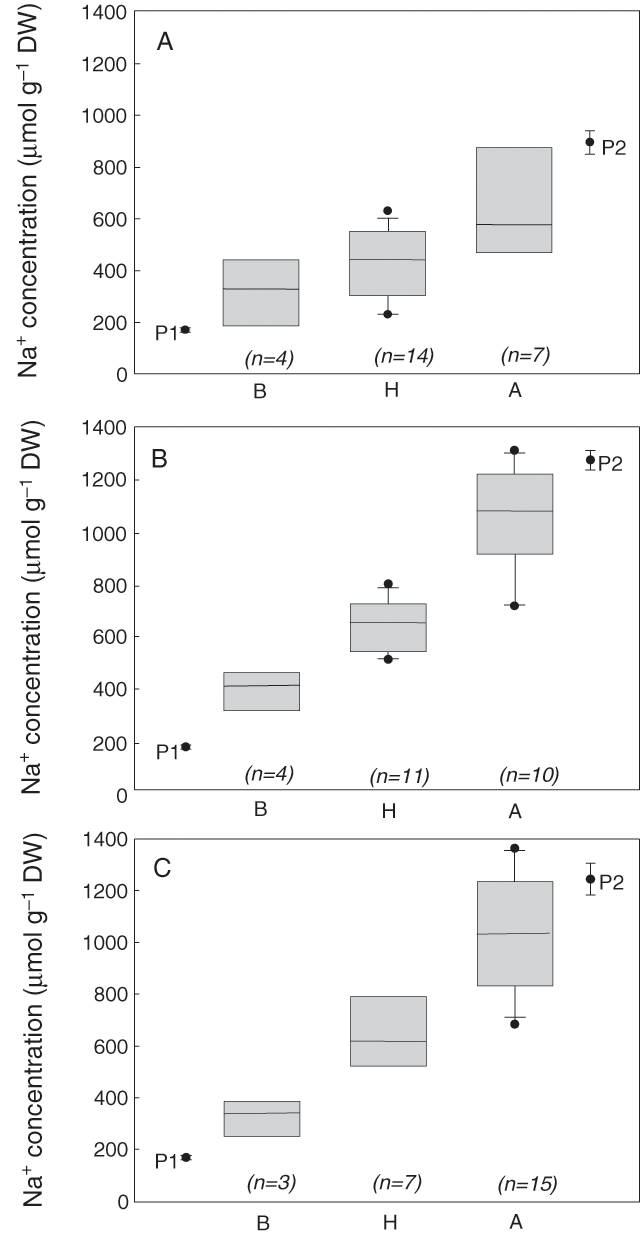
|
These results show that a breeding program based on selection for the B allele will lead to the transfer of the Na+ exclusion trait into recurrent parents of very different genetic background.
Discussion
A locus for the Na+ exclusion trait in Line 149 was successfully mapped using a QTL approach. Several AFLP, RFLP and microsatellite markers were linked to the gene(s) at the QTL, designated Nax1. The markers that mapped to the Nax1 locus have previously been mapped to chromosome 2A in hexaploid wheat. According to Roder et al. (1998) and Harker et al. (2001), Xgwm312 and Xwmc170, the two microsatellites most closely linked to the locus, map to the long arm of chromosome 2A in hexaploid wheat. Nachit et al. (2001) found markers that map to chromosome 2AL in hexaploid wheat map to a similar interval in tetraploid wheat. The Nax1 locus identified in this study maps to an approximate position between 6.0 and 14.0 cM from the centromere on chromosome 2AL.
The Nax1 locus is not homoeologous to the only QTL precisely mapped in hexaploid wheat for Na+ exclusion and its associated K+ / Na+ discrimination — the Kna1 locus. This has been mapped on chromosome 4DL (Dubcovsky et al. 1996). A homoeologous gene would map to chromosome 4AL, a possibility that we considered likely, as the diploid A genome Triticum species (T. boeticum, T. monococcum and T. urartu) have the K+ / Na+ discrimination trait (Gorham et al. 1991).
Other QTLs related to the control of Na+ transport have been reported in other species but have not been mapped with the same precision as the Kna1 or Nax1 locus. In rice, Koyama et al. (2001) identified several QTLs on chromosome 6 that accounted for leaf Na+ concentration. This chromosome also contained many QTLs for K+ / Na+ ratio, and Cl– concentration. A total of seven QTLs for Na+ concentration were identified, six on chromosome 6 and one on chromosome 4. However, none of these QTLs showed any association with similar traits in a closely related population (Flowers et al. 2000). Using different parents, Lang et al. (2001b) identified QTLs for leaf Na+ concentration on chromosome 2, and for Na+ / K+ ratio on chromosomes 1, 2, 7 and 12. In all of these cases the map position had a high degree of uncertainty. These results indicate that the markers identified were specific to the cross in which they were mapped.
QTLs associated with ‘salt tolerance’, i.e. growth in saline solution, have been reported in other species. Salt tolerance has been quantified either at germination, at the early seedling growth stage, or at later stages of plant development.
At the germination and early seedling stage, QTLs for salt tolerance have been identified in barley, tomato and Arabidopsis (Mano and Takeda 1997; Foolad 1999; Quesada et al. 2002). However, in these species, the genetic controls underlying responses to salinity at germination are different than during early seedling growth. QTLs for salt tolerance at germination were different from those for salt tolerance at the early seedling stage of growth. Further, the accessions most tolerant to germination were not the most tolerant to salt during the vegetative stage, and in fact, were sometimes the least tolerant (Mano and Takeda 1997; Foolad 1999; Quesada et al. 2002).
At the vegetative stage, QTLs for salt tolerance in barley were found on chromosomes on group 1, 4, 6 and 7 (Ellis et al. 1997). A later study by Ellis et al. (2002) with different barley parents reported QTLs for salt tolerance at the vegetative stage on chromosomes 2, 5 and 7. QTLs for shoot and root weight on chromosome 5 had the relatively high LOD score of 6.6 and 7.0 respectively. In field trials, QTLs for grain weight and grain nitrogen with high LOD scores, ranging from 8 to 21, were found on chromosomes 3, 4, 5 and 7. In all, there were 16 primary QTLs whose location had a high level of confidence, but these in total accounted for no more than 35% of the total phenotypic variation. None of these QTLs reported for any species or any crosses have been verified in genetically unrelated backgrounds. These results indicate that it is not easy to identify reliable loci for salt tolerance or any of its traits. This is possibly due to the complex nature of salinity and its effect on leaf area production, as the osmotic and salt-specific effects of soil salinity are difficult to distinguish (Munns 2002).
In rice, a microsatellite marker (RM223, on chromosome 8) was associated with salt tolerance at both the vegetative and reproductive stages (Lang et al. 2001a). The performance of F3 plants under salt stress could be predicted with greater than 80% accuracy from their marker genotypes, indicating its usefulness in a plant breeding program (Lang et al. 2001a). The physiological mechanism of this tolerance is unknown. In a different rice population, derived from two different parents, different chromosome regions were associated with salt tolerance: QTLs for shoot growth occurred on chromosome 11, for root growth on chromosome 3 and 9, and for survival on chromosomes 1, 2, 3 and 9 (Lang et al. 2001b). These findings illustrate that QTLs are often specific to the cross in which they were mapped, and useful only in populations derived from the same or closely related parents.
The QTL that mapped to chromosome 2AL in the durum landrace, Nax1, accounts for only approximately 40% of phenotypic variation in leaf Na+ concentrations of the F2 progeny of a cross between Tamaroi and Line 149. Genetic analysis of the distribution of Na+ concentration in the progeny of crosses between Line 149 and two parents with high Na+ (Tamaroi and Line 141) showed there were two genes of major effect (Fig. 1; Munns et al. 2003). However, only one major QTL was identified in the present study with the available markers. This may reflect the lack of extensive genome coverage in the genetic linkage groups generated in the mapping study. Of the initial 103 microsatellite markers distributed on all seven homoeologous groups from the A and B genomes (Table 2), polymorphism between the parental lines of the mapping family was identified with 36 markers. Thus, approximately 30% of markers were incorporated into the linkage groups.
The presence of a second gene conferring Na+ exclusion, in addition to the Nax1 locus, is consistent with the large number of individuals in the low Na+ phenotypes that were heterozygous for the gwm312 marker. While lines that were homozygous for the gwm312B allele inherited from the parental source Line 149 were consistently associated with the low-Na+ phenotype, the heterozygous state was inadequate to identify plants with similar low Na+. To account for the low-Na+ phenotypes found in heterozygous gwm312 plants, a second gene or genomic region independent of Nax1 may provide the full expression of the Na+ exclusion trait.
Despite having some understanding of the physiological basis of the trait (Munns et al. 2000; Husain et al. 2003, 2004), we are still uncertain of the precise mechanisms associated with the Na+ exclusion loci. Salt tolerance in the Tritiaceae is associated with sodium exclusion, which limits the entry of sodium into the plant and its transport to leaves, and results in high K+ / Na+ ratios in leaves. Sodium exclusion from the transpiration stream reaching the leaves is controlled at three stages: (1) by selectivity of the root cells taking up cations from the soil solution, (2) by selectivity in the loading of cations into the xylem vessels in the roots, and (3) by removal of sodium from the xylem in the upper part of the roots and the lower part of the shoot (Munns et al. 2002; Tester and Davenport 2003). As control of Na+ and K+ transport to the shoots involves three tissue types: the root cortex, the root stele, and the cells lining the xylem in both roots and shoots (Munns et al. 2002), and several membrane transport proteins (Tester and Davenport 2003), the two genes derived from Line 149 may function in different tissues in the plant, or may control different types of transport proteins. We have evidence that the mechanism controlled by one of the genes promotes retention of Na+ in the base of the shoots (RA James, S Husain, MP Lindsay, R Munns unpublished data). This mechanism in tetraploid wheat is different from that in hexaploid wheat, i.e. from the mechanism controlled by the Kna1 locus, hence we have designated the locus, Nax1 (rather than Kna2).
This study indicates that marker-assisted selection using gwm312 or wmc170 is an effective means of identifying Na+ excluding individuals without the need for a phenotypic screen. Although the Nax1 QTL accounts for only approximately 40% of the phenotypic variation, and may denote just one of the two major genes, the markers for this locus can be useful even in the absence of a marker for the second gene. The gwm312B allele is a reliable indicator of low Na+. It does not ensure selection of all individuals with the low Na+ trait, as some low-Na+ individuals were heterozygous, but selection of the homozygous B allele enriches backcrossed derived lines with the low Na+ phenotype.
The robust markers gwm312 and wmc170 have proven useful for the selection of the Na+ exclusion trait in durum wheat populations from a range of genetic backgrounds. Glasshouse trials with durum landraces of different degrees of Na+ accumulation have indicated that this Na+ exclusion trait can improve yield by 20% at moderate salinity levels (Husain et al. 2003). Trials with the backcrossed lines developed in this study are not yet complete. The introduction of sources of sodium exclusion into durum wheat may raise the salt tolerance of durum wheat to equal that of bread wheat, and provide more options to farmers in salt-affected areas.
Acknowledgments
We thank Richard James for developing germplasm stocks and breeding lines and for the phenotype data for the Tamaroi × Line 149 cross, Shazia Husain for the phenotype data and DNA from the Line 141 × Line 149 cross, and Lorraine Mason for Na+ analyses by atomic absorption spectroscopy. The study was funded by the Grains Research and Development Corporation (GRDC).
Dubcovsky J,
Santa Maria G,
Epstein E,
Luo MC, Dvořák J
(1996) Mapping of the K+ / Na+ discrimination locus Kna1 in wheat. Theoretical and Applied Genetics 2, 448–454.
(validated 5 October 2004)
Flowers TJ,
Koyama ML,
Flowers SA,
Sudhakar C,
Singh KP, Yeo AR
(2000) QTL: their place in engineering salt tolerance of rice to salinity. Journal of Experimental Botany 51, 99–106.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
(validated 5 October 2004)
Harker N,
Rampling LR,
Shariflou MR,
Hayden MJ,
Holton TA,
Morell MK,
Sharp PJ,
Henry RJ, Edwards KJ
(2001) Microsatellites as markers for Australian wheat improvement. Australian Journal of Agricultural Research 52, 1121–1130.
| Crossref | GoogleScholarGoogle Scholar |
(validated 5 October 2004)
Pitman, MG ,
and
Läuchli, A (2002). Global impacts of salinity and agricultural ecosystems. In ‘Salinity: environment–plants–molecules’. pp. 3–20. (Kluwer Academic: Dordrecht)
Quesada V,
Garcia-Martinez S,
Piqueras P,
Ponse MR, Micol JL
(2002) Genetic architecture of NaCl tolerance in Arabidopsis. Plant Physiology 130, 951–963.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rawson HM,
Richards RA, Munns R
(1988) An examination of selection criteria for salt tolerance in wheat, barley and triticale. Australian Journal of Agricultural Research 39, 759–772.
| Crossref |

Rengasamy P
(2002) Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview. Australian Journal of Experimental Agriculture 42, 351–361.
| Crossref | GoogleScholarGoogle Scholar |

Roder MS,
Korzun V,
Wendehake K,
Plaschke J,
Tixier M-H,
Leroy P, Ganal MW
(1998) A microsatellite map of wheat. Genetics 149, 2007–2023.
| PubMed |

Sambrook, J ,
Fritsch, EF ,
and
Maniatis, T (1989).
Shah SH,
Gorham J,
Forster BP, Wyn Jones RG
(1987) Salt tolerance in Triticeae: the contribution of the D genome to cation selectivity in hexaploid wheat. Journal of Experimental Botany 38, 254–269.

Tester M, Davenport R
(2003) Na+ tolerance and Na+ transport in higher plants. Annals of Botany 91, 503–527.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vos P,
Hogers R,
Bleeker M,
Reijans M, van de Lee T , et al.
(1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research 23, 4407–4414.
| PubMed |



