Rapid cell expansion and cellulose synthesis regulated by plasmodesmata and sugar: insights from the single-celled cotton fibre
Yong-Ling RuanA CSIRO Plant Industry, GPO Box 1600, Canberra, ACT 2601, Australia.
Email: yong-ling.ruan@csiro.au
B This paper originates from the Peter Goldacre Award 2005 of the Australian Society of Plant Scientists, received by the author
Functional Plant Biology 34(1) 1-10 https://doi.org/10.1071/FP06234
Submitted: 20 September 2006 Accepted: 21 November 2006 Published: 19 January 2007
Abstract
Higher plants comprise mixtures of some 40 different cell types, and this often complicates the interpretation of data obtained at the tissue level. Studies for a given cell type may provide novel insights into the mechanisms underlying defined cellular and developmental processes. In this regard, the cotton fibre represents an excellent single-cell model to study the control of rapid cell elongation and cellulose synthesis. These single cells, initiated from the ovule epidermis at anthesis, typically elongate to ~3–5 cm in the tetraploid species before they switch to intensive secondary cell wall cellulose synthesis. By maturity, more than 94% of fibre weight is cellulose. To unravel the mechanisms of fibre elongation and cellulose synthesis, two hypotheses have been examined: (a) that sucrose degradation and utilisation mediated by sucrose synthase (Sus) may play roles in fibre development and (b) that symplastic isolation of the fibre cells may be required for their rapid elongation. Reverse genetic and biochemical analyses have revealed the critical role that Sus plays in fibre initiation and early elongation. Late in development, plasma-membrane and cell wall association of Sus protein seems to be involved in rapid cellulose synthesis. Cell biology and gene expression studies showed a temporary closure of fibre plasmodesmata (PD), probably due to the deposition of callose, at the rapid phase of elongation. The duration of the PD closure correlates positively with the final fibre length attained. These data support the view that PD closure may be required for fibres to achieve extended elongation. The branching of PD towards the secondary cell wall stage is postulated to function as a molecule sieve for tight control of macromolecule trafficking into fibres to sustain intensive cellulose synthesis.
Introduction
The growth of higher plants is achieved through a series of coordinated events of cell division, expansion and cell wall synthesis by ~40 different cell types (Goldberg 1988; Martin et al. 2001). The diversity in architecture at the tissue or whole-plant level reflects, to a large degree, high level of heterogeneity in the extent of cell expansion and cell wall synthesis among different cell types (Martin et al. 2001). For example, leaf mesophyll cells usually expand only several-fold after cytokinensis and undergo primary cell wall synthesis. In contrast, other cell types, such as pollen tubes and xylem cells, can quickly elongate more than a thousand times and synthesise a massive amount of secondary cell wall polymers (Fukuda 1991; Franklin-Tong 1999; Chen et al. 2003; Andersson-Gunnerås et al. 2006). The accomplishment of the extraordinary elongation of pollen tubes is critical for the delivery of the male gametes to the ovules for double fertilisation, and the synthesis of various wall materials in the xylem provides mechanical strength for the respective tissue and a network for transport water and inorganic solutes. Understanding the mechanisms controlling rapid cell expansion and cell wall synthesis is of fundamental importance for better understanding basic biology as well as for designing strategies to improve crop productivity and stress tolerance (John 1997; Haigler et al. 2001; Martin et al. 2001; Ruan 2005).
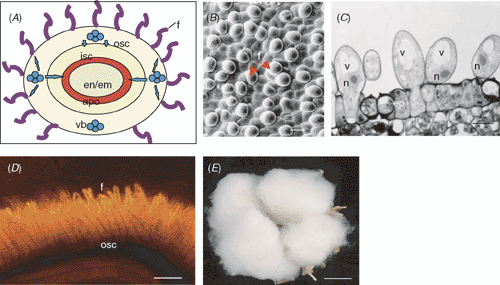
One challenge involved in the study of the molecular and cellular basis of rapid cell expansion and cell wall synthesis is the difficulties in experimentally accessing, isolating and manipulating the cell types of interest in vivo. To this end, although the pollen tube and xylem elements have been studied extensively in vitro for understanding cell elongation and cell wall biosynthesis, respectively, it is uncertain whether the phenomenon observed in vitro reflects that in vivo (Taylor and Hepler 1997). The impediment in studying these cell types in vivo is largely due to the complex anatomic structure of the tissue where those cells are located (Galbraith and Birnbaum 2006). For example, pollen tubes penetrate within the transmitting tract of the style (Taylor and Hepler 1997) and stem xylem elements are deeply embedded between the ground parenchyma cells of cortex and pith and surrounded by phloem cells (e.g. Wu et al. 2000). These inherent anatomical features make it a difficult task to isolate those specialised cell types from the surrounding tissues unless through the use of techniques such as laser capture microdissection (see Galbraith and Birnbaum 2006), which is expensive and not available to most laboratories. Thus, the developing cotton fibre represents an excellent system to study the mechanisms of rapid cell expansion and cell wall synthesis for the following reasons. First, unlike the pollen tube, which contains triple nuclei of two sperm cells plus one vegetative nucleus, complicating the genetic makeup of the system (Franklin-Tong 1999), each cotton fibre has one nucleus and does not undergo cell division during development in planta (Basra and Malik 1984; Ruan et al. 2000; Taliercio et al. 2005). Therefore, it represents a true single-cell system to study without the complexity of cell division and multicellular development (Ruan et al. 2001). Second, cotton fibres grow outwards from the seed epidermis and are, thus, readily accessible in vivo by opening up the pericarp of the fruit. Third, the rapid and synchronised growth of the fibre cells ensures a sufficient amount of samples for experimentation. Here, each cotton seed produces 10 000 to 18 000 fibre cells from its epidermis (Van’t Hof and Saha 1997; Taliercio et al. 2005) with the average density of one fibre in every 3–4 epidermal cells (Fig. 1B, C). By 10 days after anthesis (DAA), ~5–6 g of fresh fibre cells can be harvested from each fruit, and this yields sufficient amount of RNA, proteins and metabolites for various analyses (Y-L Ruan, unpublished data). This is in contrast with other cell types isolated through the laser microsampling method, which often results in very small amounts of materials (Galbraith and Birnbaum 2006). Further, developing cotton fibre has distinct and continuous developmental programs, from initiation at anthesis to rapid elongation by ~18 DAA, followed by intensive secondary cell wall cellulose synthesis from ~18 to 40 DAA before maturation and desiccation (Basra and Malik 1984; also see Fig. 1). This provides an invaluable advantage that a particular molecular or cellular event can be precisely mapped or correlated with the corresponding developmental program at the single cell level. Finally, in addition to being an excellent system for studying cell growth, cotton fibre is also the most important source of cellulose for the textile industry, with >94% of mature fibre dry weight being cellulose, a β-1,4 polymer of glucose (Basra and Malik 1984; Delmer 1999). Hence, understanding the mechanisms underlying rapid cell elongation and cellulose production, two key processes that determine cotton fibre quality and yield, could lead to the identification of target genes for genetic engineering of fibre development for the improvement of cotton productivity (John 1997; Kim and Triplett 2001; Ruan 2005).
|
This paper appraises and discusses recent progress in understanding cotton fibre elongation and cellulose synthesis, focusing on the role of plasmodesmta (PD) and sucrose metabolism mediated by sucrose synthase (Sus) in fibre development. Where appropriate, comparisons have been made with other cell systems to stimulate thinking and to conceive new hypotheses for future research.
The control of cotton fibre elongation
The unidirectional and diffusive growth of cotton fibre
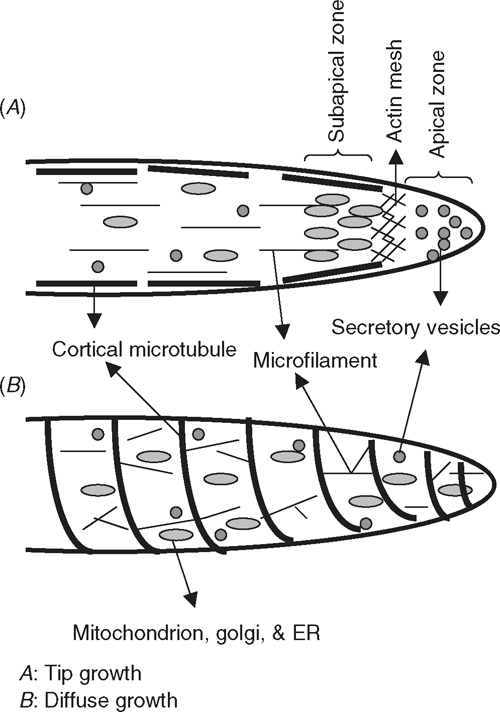
Plant cell expansion follows either diffuse growth or tip growth (Yang 1998; Martin et al. 2001). Typical unidirectional and fast-growing cells such as pollen tube, root hair and fungal hyphae, follow a tip growth pattern, where expansion is confined to the dome-shaped apex that is filled with secretory vesicles (Franklin-Tong 1999). Rapidly elongating cotton fibre cells, however, seem to grow by diffuse growth based on the following observations (also see Fig. 2). First, the elongating fibre cells lack the ‘zonenation’ phenomenon of intracellar organelles in the cell tip (Tiwari and Wilkins 1995). The concentration of endoplasmic reticulum (ER), Golgi bodies and mitochondria in the subapical region of the cell tip is a major characteristic of tip growth (Yang 1998). Second, the orientation of cortical microtubules, and, hence, that of the newly deposited cellulose microfibrils, are transversely organised to the growth axis in the cotton fibre, which provides a greater resistance to radial expansion than to a longitudinal expansion (Seagull 1990, 1992; Martin et al. 2001). Thus, in response to turgor pressure, the fibre cells elongate perpendicularly to the orientation of cellulose microfibrils, leading to unidirectional outgrowth from the seed epidermis (see Ruan 2005). In tip growth cells such as in the pollen tube, however, microtubules are arranged as bundles in parallel to the growth axis and are absent from the apical dome (Yang 1998), which may allow the apical region to be free of constriction by cellulose microfibrils and, thus, to grow readily forwards.
|
Another cytoskeleton component, microfilaments composed of actin filaments, support the intracellular trafficking of organelles and secretory vesicles (Chen et al. 2003). Actin filaments are arranged as long bundles along the length of the cells during tip growth but randomly during diffuse growth (Fig. 2). In addition, actin filaments may form a meshwork in the subapical region of tip growth cells (Franklin-Tong 1999), a phenomenon that does not exist in cotton fibre (Seagull 1990, 1992). The meshwork of actin may facilitate vesicle transport and docking within the apex and could also function as a filter to prevent the large organelles from entering the apical region, thus, creating a so called ‘clear zone’ in the tip (Martin et al. 2001). The essential role of actin in the tip growth of pollen tube has been shown by the overexpression of Rac/Rop GTPases that disrupts actin cytoskeleton and converts polar growth into isotropic growth (Chen et al. 2003). In cotton fibres, silencing the expression of a fibre-specific actin gene, GhACTIN1, reduces the amount of F-actin and inhibits fibre elongation, probably through decreasing the number of organelles (such as ER and Golgi bodies) travelling along the filamnents, and hence, the number of vesicles transported to the cell periphery for membrane and cell wall biosynthesis (Li et al. 2005).
It is not known why the polar growth of some cell types displays a tip growth feature but cotton fibre follows a pattern of diffuse growth. One possibility is that it may relate to the environment where these cells were evolved. Here, the growth of pollen tube and root hair needs to overcome the resistance imposed by the transmitting tissue of the style and soil particles, respectively. The tip growth of these cells may be advantageous in penetrating through the physical barrier encountered. In contrast, the outgrowth of cotton fibre occurs relatively ‘freely’ in the aqueous space between the seed coat and the pericarp without the need to overcome the physical resistance that the pollen tube and root hair face. Similar to a cotton fibre, the aerial protrusion of trichomes from the leaf epidermis also follows a pattern of diffuse growth (Mathur et al. 1999; Szymanski et al. 1999).
The power of symplastic isolation in fibre elongation
Cotton fibres typically elongate over 2000–3000-fold within 3 weeks after initiation. The final length attained can reach 3–5 cm in the tetraploid cotton of Gossypium hirsutum and G. barbadense, respectively. This renders the cotton fibre as the fastest growing and longest single cell known in plants (Ruan 2005). The adjacent seed coat cells, however, expand by only 5–10-fold during this period. This indicates a high degree of cell autonomy of the fibres. One mechanism for such a discrepancy in cell growth and function is the symplastic isolation between the respective tissues or cell types through the closure of PD, the plasma membrane and ER-lined cytoplasmic channels connecting plant cells (see Roberts and Oparka 2003; Zambryski 2004). To examine the symplastic continuity between fibres and underlying seed coat epidermis, a membrane-impermeant fluorescent dye, carboxyfluorescein (CF), was ester-loaded into phloem of a fruit-bearing shoot. Confocal microscopic imaging was then used to monitor whether or not the CF unloaded from the phloem of the seed coat moves into the fibres (Ruan et al. 2000). The presence of the fluorescent signals of CF in the fibre indicates that the fibre PD is open or permeable to CF import; otherwise, the PD is closed. As the molecular mass of CF is similar to sucrose, the kinetics of CF movement is likely to reflect that of endogenous solute transport (Ruan and Patrick 1995).
A systematic imaging analysis of the CF movement revealed that the fibre PD is initially open but become closed for ~5 days during the rapid phase of elongation before they reopen at ~16 DAA, at the end of elongation (Ruan et al. 2001). Further comparison among genotypes differing in fibre length shows that the duration of PD closure correlates positively with the final fibre length attained, and the closure always occurs at the onset of rapid phase of elongation (Ruan et al. 2004, 2005). These observations strongly suggest that the closure of PD in the fibre base is important for the rapid and extensive elongation of cotton fibre.
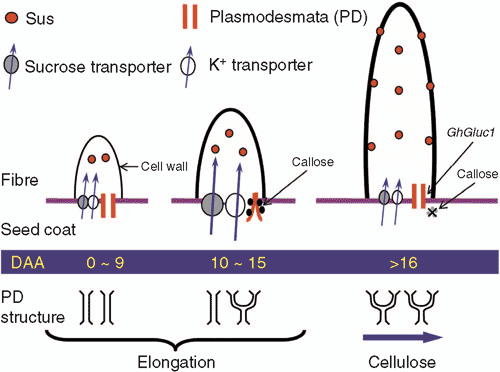
The closure of PD may provide a mechanism for the fibre cells to generate and maintain a higher turgor to drive the extensive elongation. Cell turgor is generated through the influx of water driven by the accumulation of osmotically active solutes. In elongating cotton fibre, malate, potassium and soluble sugars account for most of the osmotic potential (see Ruan 2005). Although malate is produced within the fibre through re-fixation of CO2 by phosphoenolpyruvate carboxylase (Smart et al. 1998), potassium and sucrose are imported from the underlying seed coat cells, either symplastically through the PD or apoplastically across cell wall space and plasma membrane. The closure of fibre PD would necessitate solute uptake into fibres across their plasma membranes. Indeed, transporter genes, GhSUT1 and GhKT1, which encode a H+/sucrose symporter and a potassium transporter, respectively, are maximally expressed when the PD is closed at the fibre base (Ruan et al. 2001). The high expression of these transporter genes facilitates the uptake of sucrose and potassium, hence, the generation of the observed low osmotic potential (Ruan et al. 2001). The closure of the fibre PD likely enables the estimated high turgor to be maintained to drive the elongation as long as osmotically active solutes are continuously imported to attract water (Cosgrove 1997; Pfluger and Zambryski 2001) and the cell wall is expandable through the expression of expansin genes (Ruan et al. 2000; Harmer et al. 2002). Consistent with this hypothesis is the reopening of fibre PD, which correlates with the decrease of estimated fibre turgor and the termination of the elongation process (Ruan et al. 2001).
It is possible that the hydraulic isolation of the elongating fibres from the remainder of the seed coat may be a requirement for rapid restoration and maintenance of cell turgor following cell wall relaxation through osmotic adjustment mediated by membrane transport. In addition, the hydraulic isolation would render fibre water relations independent of the vegetative plant, and that this may prevent or reduce the backflow of water from elongating fibres to leaves down a water potential difference (X-W Fang, Y-L Ruan, unpublished data).
Developmentally-reversible gating of PD has been reported in the shoot apex of birch trees (Rinne et al. 2001) and Arabidopsis (Gisel et al. 2002). The transient closure of PD correlates with dormancy in the former, and with flowering time in the later. In both cases, however, it has not been possible to locate the cellular site where the restriction and restoration of the symplastic connection occurs because of the multicellular differentiation processes in the shoot apical meristem (Gisel et al. 2002). The findings in cotton fibre (see above) provide evidence for a direct role of PD gating in plant growth at the single cell level (Pfluger and Zambryski 2001).
The molecular basis of PD gating in plants is unclear since the components that control the structure and function of PD remain virtually unknown (Roberts and Oparka 2003), and mutational screening for alteration in PD function could have lethal effects (Zambryski 2004). In some cases, the switch between symplastic and apoplastic pathways relates to the decrease or absence of PD connection (Patrick 1997) or the presence of an apoplastic barrier (e.g. Walsh et al. 2005). In cotton fibres, several studies indicate that deposition and degradation of callose, a β-1,3 polymer of glucose, may be involved in the closure and opening of PD, respectively. Here, by using a monoclonal antibody against callose and a callose-specific stain, aniline blue, the glucose polymer is specifically localised in the neck region of PD at the fibre base when PD is closed but not at the time when PD is open (Ruan et al. 2004). The expression of a fibre-specific β-1,3 glucanase gene, GhGluc1, is strong at the time of callose degradation but undetectable when callose is deposited at the fibre base, suggesting that expression of GhGluc1 may be responsible for the degradation of callose and consequently the re-opening of the fibre PD (Ruan et al. 2004). It would be of significance to examine if and how PD gating and fibre elongation are affected when GhGluc1expression is genetically altered.
The critical role of Sus in fibre elongation
As illustrated above, the closure of fibre PD coincides with the strong expression of transporter genes for the uptake of sucrose and K+ across the plasma membrane of the fibre cells (Ruan et al. 2001). To this end, the subsequent utilisation of sucrose within the fibres may play multiple roles in fibre development, from contributing to the generation of turgor potential to the supply of substrate for cellulose biosynthesis (Ruan 2005; Ruan and Chourey 2006). In most of the sink cells, sucrose is either hydrolysed by invertase into glucose and fructose or degraded by Sus into uridine 5′-diphosphate (UDP) glucose and fructose for subsequent metabolism and biosynthesis (Geigenberger and Stitt 1993; Koch 1996). A series of cellular, biochemical and molecular studies has provided strong evidence that Sus plays a critical role in cotton fibre development. During the initiation and early elongation period, sucrose moves into fibres symplastically based on imaging of CF movement and plasmolysis analyses (Ruan et al. 2000, 2001). This would render Sus as the major enzyme to degrade incoming sucrose in the cytoplasm because the activity of the second sucrose-degrading enzyme in cytosol, neutral/alkaline invertase, is nearly 10-fold lower than Sus in fibre cell (Y-L Ruan, unpublished data). Indeed, Sus protein and mRNA are abundantly and specifically localised in initiating fibres but not in the adjacent non-differentiating epidermal cells nor in the ovule epidermis of a fibre-less mutant (Nolte et al. 1995; Ruan and Chourey 1998). Consistent with a role of Sus in fibre development, microarray studies have also shown that expression of Sus, among other genes including expansin and some transcription factors, is strongly reduced in the ovule epidermis of several mutants defective in fibre initiation (Wu et al. 2006). Significantly, the suppression of Sus expression in the ovule epidermis leads to shrunken or collapsed fibre initials and the repression of fibre elongation in transgenic cotton plants (Ruan et al. 2003), demonstrating the essential role of Sus in fibre growth.
These observations are important for two reasons. First, unlike those found in other sink tissues, including maize kernel (Chourey et al. 1998), potato tubers (Zrenner et al. 1995) and tomato fruit (Chengappa et al. 1999), where a large amount of Sus activity seems to be dispensable, the initial spherical expansion and subsequent elongation of cotton fibre is sensitive to a small reduction of Sus expression (Ruan et al. 2003). Second, Sus has been previously shown to be involved mainly in the storage of protein and starch in sink tissues (Weber et al. 1997). The elongating cotton fibre, however, does not show detectable levels of these storage activities (Basra and Malik 1984). Apparently, the fast-growing cotton fibre represents a very strong sink, the activity of which depends strongly on the level of Sus expression. The biochemical basis for the critical role of Sus in fibre growth most likely relates to its ability in providing hexoses and UDPglucose to the fibre cells. A large amount of hexose could be required for the fibre cells to generate osmotic potential, hence, turgor for their protrusion above the ovule surface as well as to produce energy (ATP) and substrates for protein and membrane biosynthesis. Support for this view comes from the observation that the levels of glucose and fructose are significantly reduced in the fibre-suppressed transgenic ovules (Ruan et al. 2003). Further, Sus may control fibre development by providing UDPglucose, the immediate substrate for cell wall cellulose biosynthesis (Delmer 1999). Both of these possibilities could account for the collapsed phenotype of fibre cells when Sus is suppressed (Ruan et al. 2003). Figure 3 represents an integrated model of fibre development regulated by Sus, PD gating and branching (see below).
|
The switch from elongation to cellulose synthesis: possible role of PD branching
Cotton fibre elongation slows down dramatically at ~16 DAA and terminates by ~20 DAA, accompanying concurrently with the onset of intensive secondary cell wall cellulose synthesis (Basra and Malik 1984). The molecular basis of this developmental switch remains largely unknown, although it may involve hormonal signaling, rearrangement of the cytoskeleton and oxidative burst mediated by small GTPases (see Ruan 2005 for a recent review).
In light of the distinct features of elongation and secondary cell wall thickening of the fibre cells, it is reasonable to envisage that different mechanisms may be operative for the control of molecular trafficking between the fibres and underlying seed coat cells at the two developmental stages. Studies in other systems have shown that PD may function as a ‘molecular sieve’ in regulating intercellular movement of macromolecules including transcription factors during development and cell patterning (Cilia and Jackson 2004; Kurata et al. 2005). For example, in Arabidopsis, myeloblastosis-like transcription factors, encoded by TRY and CPC, could selectively move from trichome cells and root hair to adjacent epidermal cells via PD and act as lateral inhibitors for trichome and root hair formation, respectively (Schellmann et al. 2002). In this regard, it is noted that the structural changes of PD in cotton fibres before the onset of secondary cell wall cellulose synthesis. Here, the fibre PD are all in the simple form early in elongation, but about half become branched towards the fibre side at 10 DAA (Ruan et al. 2001). Most of the fibre PD becomes branched by 16 DAA onwards at the secondary cell wall cellulose synthesis stage (Ruan et al. 2001; also see Fig. 3). The branching of PD does not correlate with the changes of its permeability to small molecules, because the initially opened PD is closed at 10 DAA and reopens at 16 DAA for CF transport (see previous discussion). Rather, the modification of the PD structure may provide a basis for tighter control of macromolecular trafficking into and out of fibre cells, similar to those proposed for the PD connecting companion cells of phloem (Oparka and Turgeon 1999). It is postulated that the stringent control of molecular trafficking by the branched PD may be required for the secondary cell wall cellulose synthesis in fibres through, for example, blocking and facilitating the import of putative repressors and activators for cellulose synthesis, respectively. This hypothesis is supported by the observation that the timing and frequency of PD branching positively correlates with the commencement of secondary cell wall cellulose synthesis among three cotton genotypes examined (R White, RT Furbank, Y-L Ruan, unpublished data).
The transition from simple to branched PD during development has been reported from green algae (Franceschi et al. 1994) to leaves and roots of plants (Itaya et al. 1998; Zhu et al. 1998). In tobacco, for example, there is a clear structural change of PD from simple form in sink leaves to branched shape in source leaves (Ding et al. 1992; Oparka et al. 1999). Importantly, whereas the simple PD allows non-specific trafficking of green fluorescent protein (GFP) or GFP-fused protein at the molecular mass up to 50 KDa, the branched PD does not permit the passage of GFP, a molecule of 27 KDa (Oparka et al. 1999). In addition, viral movement proteins from tobacco and cucumber mosaic viruses only target and dilate the branched PD but not the simple one in tobacco leaves (Ding et al. 1992; Itaya et al. 1998). These results show not only a much reduced size exclusion limit but also possibly different molecular components (e.g. putative receptors and chaperons) of the branched PD as compared to the simple PD (Ding et al. 1992; Roberts and Oparka 2003). Further support for the suggestion that branched PD have different functions from the simple PD comes from the observation that expression of a yeast invertase in transgenic tobacco inhibits the formation of branched PD but not the simple PD (Ding et al. 1993). Such modifications in PD structure and function may be necessary for tight screening of the input and output of proteins and RNA, so that specific functions can be performed in the cells or tissues at more advanced stage of development (Lucas et al. 1993; Oparka and Turgeon 1999). The progressive branching of PD in cotton fibre development (Fig. 3) mirrors that in tobacco leaves during sink-to-source transition (Ding et al. 1992; Oparka et al. 1999). It would be of interest to examine if the size exclusion limit of the branched PD in cotton fibre is different from that of simple form and if so, whether and how it relates to the onset of secondary cell wall cellulose synthesis.
The channelling of carbon by Sus for rapid secondary cell wall cellulose synthesis
Cellulose, the most abundant renewable biomass produced on earth, accounts for 28–30% of dry matter in typical forage grasses, 42–45% of wood and more than 94% of mature cotton fibre (see Haigler et al. 2001). It is a major polymer in plant cell walls that play critical roles in the establishment of plant structure and morphogenesis (Doblin et al. 2002). Clearly, understanding how carbon is partitioned into cellulose is a critical question in both plant biology and biotechnology. Several studies discussed below point to the important role of Sus in this process.
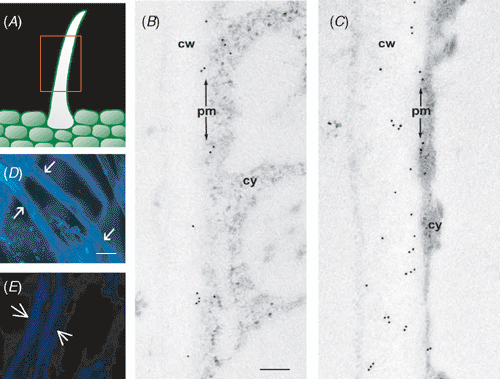
A remarkable characteristic of cotton fibre development is that at the onset of secondary cell wall formation stage, the rate of cellulose synthesis abruptly rises over 100-fold compared with the elongation and primary cell wall synthesis stage (Delmer 1999). To support this rapid cellulose synthesis, a virtually irreversible carbon sink, some 80% of total carbon entering the fibres is partitioned to cellulose (Mutsaers 1976). It is intriguing how so much imported carbon, mainly in the form of sucrose (Ruan et al. 1997), is channelled to cellulose synthesis on the plasma membrane. To this end, Amor et al. (1995) made a surprising discovery that a substantial amount of Sus, a protein traditionally thought to be exclusively located in the cytosol (reviewed by Winter and Huber 2000), is tightly associated with the plasma membranes of cotton fibre. It is estimated that sucrose entering the fibre cells is partitioned in the ratio of 3 : 1 between membrane (PM-) and soluble (S-) Sus at the secondary cell wall stage (Delmer 1999). Further immunolocalisation analyses at the electron microscopic level showed that the occurrence of PM-Sus is developmentally regulated. Here, the immuno-gold labelled PM-Sus is only sporadically present in fibres at 10 DAA (Fig. 4B) but becomes more evident at 20 DAA (Fig. 4C) when the fibres are at the elongation and secondary cell wall cellulose stages, respectively (Basra and Malik 1984). Enzyme assay for putative PM-Sus from ethylene glycol tetracetic acid-eluted pellet fraction (see Haigler et al. 2001) also shows undetectable activity in 10-day-old fibres but some 390 nmol mg–1 protein min–1 from 20-day-old fibres. It is of interest to note that in 20-day-old fibres the gold-labelled Sus particles are also abundantly present in the cell wall region of the fibre cells towards the plasma membrane side (Fig. 4C), so called exoplasmic zone (see Salnikov et al. 2003). This Sus protein is hereafter termed CW-Sus. It is unlikely that the CW-Sus is an artifact associated with sample preparation because it has also been observed in cryogenic-fixed and freeze-substituted cotton fibres harvested at the secondary cell wall stage (Salnikov et al. 2003). Cryogenic methods accurately preserve cellular structure and block molecular movement during sample processing (Nicolas and Bassot 1993). The developmental correlation of PM-/CW-Sus with the secondary cell wall cellulose synthesis indicates its role in channelling carbon from sucrose to cellulose (Delmer 1999; Haigler et al. 2001). Consistently, abolishing PM-/CW-Sus through transgenic suppression (data not shown) leads to little no or fluorescent signals of cellulose in cotton fibre at 20 DAA (Fig. 4E), which is in contrast to the abundant signals of cellulose in the wild type cotton fibre at this stage (Fig. 4D). These observations indicate that the PM-/CW-Sus plays an important role in the secondary cell wall cellulose synthesis of cotton fibre.
|
Evidence that supports the role of Sus in cell wall cellulose synthesis also comes from studies in other systems. For example, in the tension wood of poplar trees, two Sus genes PttSUS1 and PttSUS2 are among the most highly expressed genes and coordinately up-regulated with two cellulose synthase genes PttCESA1 and PttCESA1 during the secondary cell wall cellulose synthesis stage of developing xylem (Hertzberg et al. 2001; Andersson-Gunnerås et al. 2006). Sus is also highly expressed in wall thickening transfer cells and endosperm cells in the developing seed of maize (Chen and Chourey 1989) and cotton (Ruan et al. 1997). Mutation of one Sus gene, Sh1, in maize de-generates some endosperm cells, which have extremely thin and fragile cell walls (Chen and Chourey 1989). A similar cell wall phenotype is also seen in transgenic cotton plants, where Sus is repressed in the endosperm (Ruan et al. 2003). These observations indicate a role of Sus in cell wall synthesis. Although the precise intracellular location of Sus is unknown in these cells, it is proposed that the cell wall synthesis is dependent on PM-Sus (Haigler et al. 2001). Consistent with this view is that the level of PM-Sus in maize endosperm peaked during cellularisation and significantly dropped at the stage of starch synthesis (Carlson and Chourey 1996). In tracheary elements differentiated from mesophyll cells of Zinnia elegans L., Sus is abundantly localised near the plasma membrane underlying wall thickening and near patterned aggregates of cellulose synthase (Salnikov et al. 2001), suggesting to a direct linkage between PM-Sus and cell wall cellulose synthesis.
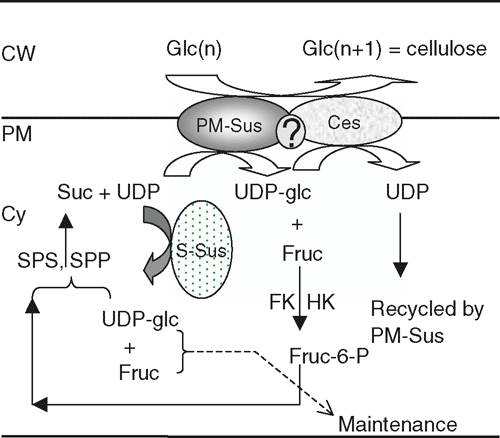
How PM- or CW-Sus regulates cellulose synthesis is a subject being intensively studied by several laboratories. Data from cotton fibre (Amor et al. 1995; Haigler et al. 2001; Salnikov et al. 2003; Fig. 4) and developing xylem (Andersson-Gunnerås et al. 2006) collectively support the hypothesis that the PM-Sus may form a complex, either directly or indirectly, with the cellulose synthase on the plasma membrane to channel carbon directly from sucrose to cellulose as originally proposed by Delmer (1999). Coupling between the PM-Sus and glucan synthase has the advantage of (a) promoting synthesis of cellulose from sucrose with no additional energy input, (b) avoiding competition for use of UDPglucose by other pathways, and (c) allowing immediate recycling of UDP, a compound that inhibits the reaction catalysed by cellulose synthase (see Haigler et al. 2001 for a recent review). In this model, fructose released from PM-Sus could be phosphorylated by fructokinase or kexokinase (Andersson-Gunnerås et al. 2006) and then recycled to sucrose by the joint action of sucrose phosphate synthase and sucrose phosphate phosphatase, whereas the energy and hexoses required for the maintenance of cell growth is provided by the soluble Sus in the cytosol. Figure 5 represents a schematic model on carbon partitioning to cellulose synthesis mediated by Sus.
|
The mechanism(s) of the membrane association of Sus is largely unknown, but might involve de-phosphorylation of the serine residue(s) at the N-terminal of the protein and interaction with actin (Winter and Huber 2000). In maize, phosphorylation of Ser 15 of the Sus1 protein promotes a soluble phase localisation of Sus, and de-phosphorylation tends to stimulate membrane association (Winter and Huber 2000). The phosphorylation of Ser 15 is developmentally regulated in maize leaves and affects N-terminal conformation in a way that may stimulate the catalytic activity of Sus and influence membrane association (Hardin et al. 2004). Similar to that in maize, the membrane-Sus protein in mature soybean nodules is also hypophosphorylation specifically on Ser 11 relative to the soluble form (Komina et al. 2002). However, in cotton fibres, the level of 32P-labelling of Sus is similar between membrane and soluble fractions and the role of de-phosphorylation in plasma membrane association is yet to be shown (Haigler et al. 2001). Nothing is known of how Sus moves to the cell wall region (Fig. 4; Haigler et al. 2001; Salnikov et al.2003). This is particularly puzzling because the Sus protein does not have an identifiable secretory signal sequence. Further work in this area may reveal novel mechanisms of Sus in intracellular partitioning and cell wall cellulose synthesis.
Conclusions and future perspectives
Recent studies discussed in this paper establish a mechanistic model on the control of cotton fibre elongation and cellulose synthesis, mediated by PD gating, branching and expression of Sus and transporters for the uptake of sucrose and K+ (Fig. 3). The model not only provides novel insights into the understanding of rapid cell expansion and cellulose synthesis, but also raises new issues for further studies. Some of the immediate questions are: how important is the PD closure for fibre elongation? For example, will the fibre elongation be affected when the fibre PD is dilated by some virus movement proteins? Are there any differences in the size exclusion limit and selectivity of molecular trafficking between simple and branched PD? How does Sus protein associate with plasma membrane and move to the cell wall? What will happen to fibre development once Sus is overexpressed? In addition to the issues relating to PD and sucrose utilisation, many other equally important biological questions can also be addressed in this system. For example, the large size of the fibre cell indicates that sufficient water needs to be imported and maintained to sustain the elongation process. What is the cellular and molecular basis of water influx into and possibly efflux from the fibre cells? How does the water movement relate to fibre cell elongation, desiccation and maturation? What is the signaling pathway that triggers the transition from elongation to secondary cell wall cellulose synthesis? Answers to these questions will come from studies using multidisciplinary approaches with strategies ranging from hypothesis-driven research to global gene expression studies at RNA, protein and metabolite levels. There is no doubt that developing cotton fibre represents an excellent system in which mechanisms underlying rapid cell expansion and cellulose synthesis can be addressed at the single cell level in defined developmental stages.
Acknowledgements
I thank Rosemary White and Celia Miller for conducting the electron microscopic localisation of Sus protein shown in Fig. 4 and many colleagues for their support. The research in the author’s group was funded, in part, by Australian Cotton Research and Development Corporation.
Amor Y,
Haigler CH,
Johnson S,
Wainscott M, Delmer DP
(1995) A membrane-associated form of Sus and its potential role in synthesis of cellulose and callose in plants. Proceedings of the National Academy of Sciences USA 92, 9353–9357.
| Crossref | GoogleScholarGoogle Scholar |

Andersson-Gunnerås S,
Mellerowicz EJ,
Love J,
Segerman B,
Ohmiya Y,
Coutinho PM,
Nilsson P,
Henrissat B,
Morotz T, Sundberg B
(2006) Biosynthesis of cellulose-enriched tension wood in Populus: global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary cell wall biosynthesis. The Plant Journal 45, 144–165.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Basra A, Malik CP
(1984) Development of the cotton fibre. International Review of Cytology 89, 65–113.

Blanton RL
(1993) Prestalk cells in monolayer cultures exhibit two distinct modes of cellulose synthesis drung stalk cell differentiation in Dictyostelium. Development 119, 703–710.

Carlson SJ, Chourey PS
(1996) Evidence for plasma membrane-associated form of sucrose synthase in maize. Molecular and General Genetics 252, 303–310.
| PubMed |

Chen CYH,
Cheung AY, Wu H-M
(2003) Actin-depolymerization factor mediates Rac/Rop GTPase-regulated pollen tube growth. The Plant Cell 15, 237–249.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chen Y-C, Chourey PS
(1989) Spatial and temporal expression of the two sucrose synthase genes in maize: immunohistological evidence. Theoretical and Applied Genetics 78, 553–559.
| Crossref | GoogleScholarGoogle Scholar |

Chengappa S,
Guilleroux M,
Phillips W, Shields R
(1999) Transgenic tomato plants with decreased sucrose synthase are unaltered in starch and sugar accumulation in the fruit. Plant Molecular Biology 40, 213–221.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chourey PS,
Taliercio EW,
Carlson SJ, Ruan YL
(1998) Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Molecular and General Genetics 259, 88–96.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cilia ML, Jackson D
(2004) Plasmodesmata form and function. Current Opinion in Cell Biology 16, 500–506.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cosgrove DJ
(1997) Relaxation in a high-stress environment: the molecular bases of extensible cell walls and cell enlargement. The Plant Cell 9, 1031–1041.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ding B,
Haudenshield JS,
Hull RJ,
Wols S,
Beachy RN, Lucas WJ
(1992) Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in treansgenic tobacco plants. The Plant Cell 4, 915–928.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ding B,
Haudenshield JS,
Willmitzer L, Lucas WJ
(1993) Correlation between arrested secondary plasmodesmatal development and onset of accelerated leaf senescence in yeast invertase transgenic tobacco plants. The Plant Journal 4, 179–189.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Doblin MS,
Kurek I,
Jacon-Wilk D, Delmer DP
(2002) Cellulose biosynthesis in plants: from genes to rosettes. Plant and Cell Physiology 43, 1407–1420.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Franceschi VR,
Ding B, Lucas WJ
(1994) Mechanism of plasmodesmata formation in characean algae in relation to evolution of intercellular communication in higher plants. Planta 192, 347–358.
| Crossref | GoogleScholarGoogle Scholar |

Franklin-Tong VE
(1999) Signaling and modulation of pollen tube growth. The Plant Cell 11, 727–738.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fukuda H
(1991) Tracheary element formation as a model system of cell differentiation. International Review of Cytology 136, 289–332.

Galbraith DW, Birnbaum K
(2006) Global studies of cell type-specific gene expression in plants. Annual Review of Plant Biology 57, 451–475.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Geigenberger P, Stitt M
(1993) Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 189, 329–393.
| Crossref | GoogleScholarGoogle Scholar |

Gisel A,
Hempel FD,
Barella S, Zambryski PC
(2002) Leaf-to-shoot apex movement of symplastic tracer is restricted coincident with flowering in Arabidopsis. Proceedings of the National Academy of Sciences USA 99, 1713–1717.
| Crossref | GoogleScholarGoogle Scholar |

Goldberg RB
(1988) Plants: novel developmental processes. Science 240, 1460–1467.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Haigler CH,
Ivanova-Datcheva M,
Hogan PS,
Salnikov VV,
Hwang S,
Martin K, Delmer DP
(2001) Carbon partitioning to cellulose synthesis. Plant Molecular Biology 47, 29–51.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hardin SC,
Winter H, Huber SC
(2004) Phosphorylation of the amino terminus of maize sucrose synthase in relation to membrane association and enzyme activity. Plant Physiology 134, 1427–1438.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Harmer SE,
Orford SJ, Timmis JN
(2002) Characterization of α-six expansin genes in Gossypium hirsutum (upland cotton). Molecular Genetics and Genomics 268, 1–9.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hertzberg M,
Aspeborg H,
Schrader J,
Andersson A, Erlandsson R , et al.
(2001) A transcriptional roadmap to wood formation. Proceedings of the National Academy of Sciences USA 98, 14732–14737.
| Crossref | GoogleScholarGoogle Scholar |

Itaya A,
Woo Y-M,
Masuta C,
Bao Y,
Nelson R, Ding B
(1998) Developmental regulation of intercellular protein trafficking through plasmodesmata in tobacco leaf epidermis. Plant Physiology 118, 373–385.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

John ME
(1997) Cotton crop improvement through genetic engineering. Critical Reviews in Biotechnology 17, 185–208.

Kim H-J, Triplett BA
(2001) Cotton fibre growth in planta and in vitro. Models for plant cell elongation and cell wall biogenesis. Plant Physiology 127, 1361–1366.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Koch KE
(1996) Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 509–540.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Komina O,
Zhou Y,
Sarath G, Chollet R
(2002) In vivo and in vitro phosphorylation of membrane and soluble forms of soybean nodule sucrose synthase. Plant Physiology 129, 1664–1673.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kurata T,
Okada K, Wada T
(2005) Intercellular movement of transcription factors. Current Opinion in Plant Biology 8, 600–605.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Li XB,
Fan XP,
Wang XL,
Cai L, Yang WC
(2005) The cotton ACTIN1 gene is functionally expressed in fibres and participates in fibre elongation. The Plant Cell 17, 859–875.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lucas WJ,
Ding B, Van der Schoot C
(1993) Plasmodesmata and the supracellular nature of plants. New Phytologist 125, 435–476.
| Crossref | GoogleScholarGoogle Scholar |

Martin C,
Bhatt K, Baumann K
(2001) Shaping in plant cells. Current Opinion in Plant Biology 4, 540–549.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mathur J,
Spielhofer P,
Kost B, Chua NH
(1999) The actin cytoskeleton is required to elaborate and maintain spatial patterning during cell morphogenesis in Arabidopsis thaliana. Development 126, 5559–5568.
| PubMed |

Mutsaers HJ
(1976) Growth and assimilate converson of cotton bolls (Gossypium hirsutum L.). I. Growth of fruits and substrate demand. Annals of Botany 40, 300–315.

Nicolas TN, Bassot JM
(1993) Freeze substitution after fast-freeze fixation in preparation for immunocytochemistry. Microscopy Research and Technique 24, 474–487.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nolte KD,
Hendrix DL,
Radin JW, Koch KE
(1995) Sucrose synthase localization during initiation of seed development and trichome differentiation in cotton ovules. Plant Physiology 109, 1285–1293.
| PubMed |

Oparka K, Turgeon R
(1999) Sieve elements and companion cells- traffic control centers of phloem. The Plant Cell 11, 739–750.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Oparka KJ,
Roberts AG,
Boevink P,
Cruz SS,
Roberts I,
Pradel KS,
Imlau A,
Kotlizky G,
Sauer N, Epel B
(1999) Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97, 743–754.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Patrick JW
(1997) Sieve element unloading and post-sieve element transport. Annual Review of Plant Physiology and Plant Molecular Biology 48, 191–222.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pfluger J, Zambryski PC
(2001) Cell growth: the power of symplastic isolation. Current Biology 11, R436–R439.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rinne PL,
Kaikuranta PM, van der Schoot C
(2001) The shoot apical meristem restores its symplasmic organization during chilling-induced release from dormancy. The Plant Journal 26, 249–264.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Roberts AG, Oparka KJ
(2003) Plasmodesmata and the control of symplastic transport. Plant, Cell and Environment 26, 103–124.
| Crossref | GoogleScholarGoogle Scholar |

Ruan Y-L
(2005) Recent advances in understanding cotton fibre and seed development. Seed Science Research 15, 269–280.
| Crossref | GoogleScholarGoogle Scholar |

Ruan Y-L, Patrick JW
(1995) The cellular pathway of postphloem sugar transport in developing tomato fruit. Planta 196, 434–444.
| Crossref | GoogleScholarGoogle Scholar |

Ruan Y-L, Chourey PS
(1998) A fibreless seed mutation in cotton is associated with lack of fibre cell initiation in ovule epidermis and alterations in sucrose synthase expression and carbon partitioning in developing seeds. Plant Physiology 118, 399–406.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ruan Y-L,
Chourey PS,
Delmer PD, Perez-Grau L
(1997) The differential expression of sucrose synthase in relation to diverse patterns of carbon partitioning in developing cotton seed. Plant Physiology 115, 375–385.
| PubMed |

Ruan Y-L,
Llewellyn DJ, Furbank RT
(2000) Pathway and control of sucrose import into initiating cotton fibres. Australian Journal of Plant Physiology 27, 795–800.

Ruan Y-L,
Llewellyn DJ, Furbank RT
(2001) The control of single-celled cotton fibre elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin. The Plant Cell 13, 47–63.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ruan Y-L,
Llewellyn DJ, Furbank RT
(2003) Suppression of sucrose synthase expression represses cotton fibre cell initiation, elongation and seed development. The Plant Cell 15, 952–964.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ruan Y-L,
Xu S-M,
White R, Furbank RT
(2004) Genotypic and developmental evidence for the role of plasmodesmatal regulation in cotton fibre elongation mediated by callose turnover. Plant Physiology 136, 4104–4113.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ruan Y-L,
Llewellyn DJ,
Furbank RT, Chourey PS
(2005) The delayed initiation and slow elongation of fuzz-like short fibre cells in relation to altered patterns of sucrose synthase expression and plasmodesmata gating in a lintless mutant of cotton. Journal of Experimental Botany 56, 977–984.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Salnikov VV,
Grimson MJ,
Delmer DP, Haigler CH
(2001) Sucrose synthase localizes to cellulose synthesis sites in tracheary elements. Phytochemistry 57, 823–833.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Salnikov VV,
Grimson MJ,
Seagull RW, Haigler CH
(2003) Localization of sucrose synthase and callose in freeze-substituted secondary wall-stage cotton fibres. Protoplasma 221, 175–184.
| PubMed |

Schellmann S,
Schnittger A,
Wada T,
Okada K,
Beermann A,
Thumfahrt J,
Jürgens G, Hülskamp M
(2002) TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO Journal 21, 5036–5046.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Seagull R
(1990) The effect of microtuble and microfilament disrupting agents on cytoskeletal arrays and wall deposition in developing cotton fibres. Protoplasma 159, 44–59.
| Crossref | GoogleScholarGoogle Scholar |

Seagull R
(1992) A quantitative electron microscopic study of changes in microtuble arrays and wall microfibril orientation during in vitro cotton fibre development. Journal of Cell Science 101, 561–577.

Smart LB,
Vojdani F,
Maeshima M, Wilkins TA
(1998) Genes involved in osmoregulation during turgor-driven cell expansion of developing cotton fibres are differentially regulated. Plant Physiology 116, 1539–1549.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Szymanski DB,
Marks MD, Wick SM
(1999) Organized F-actin is essential for normal trichome morphogenesis in Arabidopsis. The Plant Cell 11, 2331–2347.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Taliercio E,
Hendrix B, Stewart JM
(2005) DNA content and expression of genes related to cell cycling in developing Gossypium hirtutum (Malvaceae) fibres. American Journal of Botany 92, 1942–1947.

Taylor LP, Hepler PK
(1997) Pollen germination and tube growth. Annual Review of Plant Physiology and Plant Molecular Biology 48, 461–491.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tiwari SC, Wilkins TA
(1995) Cotton (Gossypium hirsutum) seed trichomes expand via diffuse growing mechanism. Canadian Journal of Botany 73, 746–757.

Van’t Hof J, Saha S
(1997) Cotton fibers can undergo cell division. American Journal of Botany 84, 1231–1235.
| Crossref | GoogleScholarGoogle Scholar |

Walsh KB,
Russell CS, Brown SM
(2005) The anatomy of the pathway of sucrose unloading within the sugarcane stalk. Functional Plant Biology 32, 367–374.
| Crossref | GoogleScholarGoogle Scholar |

Weber H,
Borisjuk L, Wobus U
(1997) Sugar import and metabolism during seed development. Trends Plant Science 22, 169–174.
| Crossref | GoogleScholarGoogle Scholar |

Winter H, Huber SC
(2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activities of key enzymes. Critical Reviews in Plant Sciences 19, 31–67.
| Crossref | GoogleScholarGoogle Scholar |

Wu L,
Chandrashekhar PJ, Chiang VL
(2000) A xylem-specific cellulose synthase gene from aspen (Populus tremuloides) is responsive to mechanical stress. The Plant Journal 22, 495–502.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wu YR,
Machado AC,
White RW,
Llewellyn DJ, Dennis ES
(2006) Expression profiling identifies genes expressed early during lint fibre initiation in cotton. Plant and Cell Physiology 47, 107–127.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Yang ZB
(1998) Signaling tip growth in plants. Current Opinion in Plant Biology 1, 525–530.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zambryski PC
(2004) Cell-to-cell transport of proteins and fluorescent tracers via plasmodesmata during plant development. Journal of Cell Biology 164, 165–168.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhu T,
Lucas WJ, Rost TL
(1998) Directional cell-to-cell communication in the Arabidopsis root apical meristem. I. An ultra-structural and functional analysis. Protoplasma 203, 35–47.
| Crossref | GoogleScholarGoogle Scholar |

Zrenner R,
Salanoubat M,
Willimitzer L, Sonnewald U
(1995) Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). The Plant Journal 7, 97–107.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



