Functional evolution of photochemical energy transformations in oxygen-producing organisms
John A. RavenA Division of Plant Sciences, University of Dundee at SCRI, Invergowrie, Dundee DD2 5DA, UK. Email: j.a.raven@dundee.ac.uk
This paper is part of an ongoing series: ‘The Evolution of Plant Functions’.
Functional Plant Biology 36(6) 505-515 https://doi.org/10.1071/FP09087
Submitted: 6 April 2009 Accepted: 21 April 2009 Published: 1 June 2009
Abstract
Chlorophyll a is the photochemical agent accounting for most oxygenic photosynthesis, that is, over 99.9% of photosynthetic primary activity on Earth. The spectral and energetic properties of chlorophyll a can, at least in part, be rationalised in terms of the solar spectral output and the energetics of oxygen production and carbon dioxide reduction with two photochemical reactions. The long wavelength limit on in vivo chlorophyll a absorption is probably close to the energetic limit: longer wavelengths could not support a high rate and efficiency of oxygenic photosynthesis. Retinal, a β-carotene derivative that is the chromophore of rhodopsin, acts not only as a sensory pigment, but also as an ion-pumping photochemical transducer. Both sensory and energy-transforming rhodopsins occur in oxygenic phototrophs, although the extent of expression and the function of the latter are not well understood.
Additional keywords: chlorophyll a, photosynthesis, retinal.
Introduction
Almost all photochemical energy conversion on Earth is carried out by porphyrin cyclic tetrapyrrols in the form of chlorin, as opposed to the porphyrin of, for example, haem (cytochrome and haemoglobin) (Björn et al. 2009). Almost all oxygenic (i.e. oxygen-producing) organisms, that is, cyanobacteria, algae and embryophytic plants, use chlorophyll a for their photochemistry: a few cyanobacteria use chlorophyll d (Björn et al. 2009). These organisms that use water as their electron donor use two photochemical reactions in series (Hill and Bendall 1960) to move electrons to NADP+ and then to CO2, and pump H+ across the photosynthetic membrane: the ratio of absorbed photons used : electrons : H+ is 2 : 1 : 3 (Allen 2003, 2004; Falkowski and Raven 2007). These organisms account for over 99.9% of photosynthetic primary productivity on Earth (Raven 2009). Their two photochemical reactions have a common ancestor, and are related to the two types of photoreaction centres that occur in the photosynthetic bacteria mentioned in the next paragraph.
Anoxygenic photosynthetic organisms are all bacteria that use bacteriochlorophylls in their photochemistry, with the exception of an anoxygenic diazotrophic cyanobacterium, which uses chlorophyll a (Zehr et al. 2008). Bacteriochlorophylls are biosynthetic derivatives of chlorophylls (Gomez et al. 2007). Anoxygenic photosynthesis only accounts for ~0.1% of aquatic primary productivity, and 0.05% of total primary productivity, on Earth today (Raven 2009). In addition to autotrophy, many bacteria expressing bacteriochlorophylls have photoheterotrophy as an alternative to photoautotrophy or, often, the only possibility for growth in the light because they lack enzymes permitting autotrophic inorganic carbon assimilation (Raven 2009). This is the case for the many ‘classic’ photosynthetic bacteria whose photosynthetic apparatus only functions in the absence of oxygen, as well as for the aerobic anoxygenic photosynthetic bacteria that share the photic zone with oxygenic phytoplankton in marine and freshwater habitats (Raven 2009). Individual photosynthetic bacteria only have one type of photochemical reaction centre. The photosynthetic proteobacteria and green non-sulfur bacteria have a type II reaction centre resembling the reaction centre in oxygenic organisms that oxidises water and reduces plastoquinone, although the photosynthetic bacterial photochemistry cannot oxidise water. Green sulfur bacteria and heliobacteria have a type I reaction centre resembling the reaction centre in oxygenic organisms that oxidises reduced plastocyanin or cytochrome c 6 and reduces ferredoxin and thus NADP+. This distribution of reaction centre types appears to need horizontal gene transfer. How these two light reaction systems in oxygenic organisms evolved is considered by Allen (2005) and Allen and Martin (2007).
Rhodopsins are associations of the chromophore retinal with the polypeptide opsin. Type II rhodopsins have been known for some time in metazoans where they act as photoreceptors. Type I sensory rhodopsins are known from Archaea, Bacteria and some non-metazoan eukaryotes, and energy-transforming Type I rhodopsins occur in Archaea and Bacteria. Recently, energy-transforming in addition to sensory rhodopsins have been found in an oxygenic cyanobacterium (Miranda et al. 2009) and a green alga (Tsunoda et al. 2006).
This paper examines aspects of the functional evolution of photochemistry, light harvesting and related bioenergetics in oxygen-evolving organisms, and compares the predominant chlorophyll-based energy-transforming photochemistry with the much less common retinal-based energy-transforming photochemistry.
Chlorophyll-based photochemistry
Why chlorophyll a in photochemistry?
Björn et al. (2009) give an excellent overview of the properties of chlorophyll a that make it uniquely suitable among the chlorophylls to act as the photochemical agent in both types of photoreaction of oxygenic photoautotrophs. It is important to note that neither Björn et al. (2009) nor the present paper attempt to answer the question ‘why chlorophyll?’ in the context of autotrophy based on photochemistry on Earth. ‘Why chlorophyll’ is a question whose answer lies much earlier in evolution than the origin of oxygenic photosynthesis and, perhaps, even energy-transforming photochemistry in biology if the original role of chlorophylls associated with proteins was in the non-sacrificial dissipation of excited states caused by the absorption of UV radiation by the protein (see Mulkidjanian and Junge 1997).
Among the combination of desirable qualities of chlorophyll a cited by Björn et al. (2009) is the capacity, depending on the protein environment, to photochemically generate a radical cation (strong oxidant) at a redox potential of +1 V at the oxidising end of PSII, and a radical anion (strong reductant) at a redox potential of –1 V at the reducing end of PSI. In addition, chlorophyll a can be redox silent, as in the case of light-harvesting antennae where chlorophyll a is invariably associated with carotenoids, usually with one or more other chlorophylls sensu lato (chlorophyll b, chlorophylls c and Mg 2,4(or 3,8)divinyl, 2,4(or 3,8)desethyl-phaeoporphyrin a 5 monomethyl ester, and less commonly with phycobilins) (Larkum 2003, 2006, 2007; Björn et al. 2009). An additional desirable quality is a large system of conjugated bonds and an asymmetry of the π-bond system, which gives quite large red absorption relative to that in the blue.
The redox reactions mentioned above involve excited states of the sort produced by the absorption of red photons; absorption in the blue or other shorter wavelengths becomes this red excitation within picoseconds via a process of internal conversion (Björn et al. 2009). Larkum (2006), in reviewing the evolution of chlorophylls, synthesises information showing the relatively high attenuation coefficient of water in the red relative to the blue-green and particularly the blue region, with intermediate attenuation in the UV-B. Before global oxygenation and stratospheric ozone screening UV, photosynthetic organisms would have had to occur at depth in the water body where they would obtain adequate photosynthetically active radiation in the blue and blue-green while screening out UV-B (Raven 1987). In contrast, more red radiation might have been available to early oxygenic photoautotrophs if the UV-B screening were carried out by organic or inorganic components of the environment in, for example, microbial mats, including stromatolites, permitting the photosynthetic organisms to live closer to the ocean surface (Raven et al. 2008).
Chlorophyll a and oxygen production
The use of water as the electron donor in photosynthesis requires a photochemically generated oxidant that has a more positive redox potential than that of the oxygen–water couple (+0.81 V). The redox potential difference between the chlorophyll a form that is the photochemically generated primary oxidant (P680 +) and the initial photochemical reductant (Phaeophytin a −) of PSII is within not more than 7% of the maximum redox potential difference that could be generated by a 680-nm photon (Björn et al. 2009). There is a somewhat larger difference (~14–17%) for the PSI redox span between P700 + (the oxidised form of the photochemically active chlorophyll a) and the initial photoreductant chlorophyll a −. The maximum photon yield of PSII is correspondingly smaller than that of PSI: because equal numbers of electrons are transported through PSII and PSI in non-cyclic electron transport, the total per cell antenna size of PSII is correspondingly greater than that of PSI (e.g. Johnsen and Sakshaug 2007; Hancke et al. 2008). There are significant variations in antenna size on a reaction centre basis and on the ratio of PSI : PSII reaction centres (Strzepek and Harrison 2004; Falkowski and Raven 2007) making up the excess of light harvesting for photoreaction II relative to photoreaction I. This analysis does not take into account any need for PSI in cyclic electron flow in parallel with non-cyclic electron flow (Allen 2003; Johnson 2004; Falkowski and Raven 2007; Raven et al. 2008), but the measured difference in antenna sizes is robust.
A topic closely related to the overall energetics of the primary photochemical reaction of PSII is the extent to which the extraction of electrons from water can be thermodynamically inhibited by high oxygen (and proton) concentrations. Evidence consistent with thermodynamic inhibition of (a) reaction(s) in the oxygen-evolving pathway was presented by Clausen et al. (2005), Clausen and Junge (2008a , 2008b ) and Haumann et al. (2008). However, Kolling et al. (2009) could find no evidence of such thermodynamic inhibition, and Raven and Larkum (2007) could find no data on photosynthesis at high, but ecologically and palaeontologically relevant, oxygen concentrations in the physiological and ecological literature that require thermodynamic inhibition of oxygen evolution for their explanation.
The other product of electron extraction from water is protons (hence positive charge) that are deposited on the same side, the thylakoid lumen, of the photosynthetic membrane as oxygen is released. Unlike oxygen production there are other reactions depositing protons in the same compartment: these reactions are involved in plastoquinol oxidation and functioning of the cytochrome b 6–f complex. High incident irradiances mean that the potential for proton pumping in water oxidation and the electron flow through plastoquinol and the cytochrome b 6–f complex can exceed the capacity for backflux through CF0–CF1 ATP synthase, depending on the limitation of electron flow by the rate of NADPH re-oxidation and the capacity for pseudocyclic electron transport (the water–water cycle) and restrictions on phosphorylation by the supply of ADP and phosphate to the CF1 component.
In general, redox-driven proton pumping causes a decrease in lumen pH at high irradiances, permitting the quenching of excess excitation energy by two mechanisms of non-photochemical quenching (NPQ). One is the activation of the xanthophyll cycle de-epoxidases (in organisms with xanthophylls cycles, i.e. all but cyanobacteria and most red algae); the other is the direct interaction of protons with PsbS protein when this is present (Falkowski and Raven 2007). There is still debate about the regulation of the proton gradient across the thylakoid membrane (Kramer et al. 2003, 2004; Johnson 2004), and particularly the role of cyclic electron transport in NPQ. Whatever the resolution of this debate, it is clear that the earlier view that the transthylakoid proton electrochemical potential difference in vivo was almost entirely a pH difference is incorrect and that there is a significant component of inside-positive electrical potential difference (Kramer et al. 2003). This provides for greater possibilities of relatively independent regulation of photosphorylation and NPQ.
The outcome of these discussions is that there is no agreement as to the occurrence of thermodynamic inhibition of oxygen evolution by high oxygen concentrations. Inhibition of non-cyclic electron flow by increased proton electrochemical difference across the thylakoid membrane occurs, but it is not clear whether this is primarily an effect on water oxidation or on the cytochrome b 6–f complex. Overall, the case has not yet been made for the thermodynamic inhibition of water oxidation under natural conditions as a result of an insufficiently positive redox potential of P680 +.
Oxygen production involving other (bacterio) chlorophylls?
Björn et al. (2009) point out that one oxygenic organism is known to use a chlorophyll other than chlorophyll a in photochemistry: the cyanobacterium Acaryochloris uses chlorophyll d in photochemical reactions generating oxidised species where chlorophyll a is normally used (Larkum and Kuhl 2005). For PSI reaction centres the donor is chlorophyll d, although the electron acceptor in chlorophyll a, and the primary photochemistry of PSII, uses chlorophyll d as the oxidised species, even though chlorophyll a is also involved and the initial electron acceptor is phaeophytin a rather than phaeophytin d (Cser et al. 2008; Mimuro et al. 2008; Ohashi et al. 2008; Björn et al. 2009). The lower energy available from excitation at longer wavelengths in the reaction centres of Acaryochloris means that energy can only be trapped over a smaller redox range between primary oxidant and primary reductant if the probability of a back reaction is not to be increased. The available evidence suggests that the redox potential of oxidised P713 (photochemically active chlorophyll d of PSII) in Acaryochloris is similar to that of P680, although the redox potential of the primary reductant (and secondary reductants?) in Acaryochloris may be higher (Cser et al. 2008; Mimuro et al. 2008; Ohashi et al. 2008; Björn et al. 2009). For PSI much of the evidence favours similar redox potentials for oxidised P740 (photochemically active chlorophyll d of PSI) as for P700, with the expectation that the primary reductant would have a higher redox potential; further work is needed here.
The maximum specific growth rate that has been achieved in culture for Acaryochloris is low relative to the rates found for related cyanobacteria of a similar cell size (Swingley et al. 2005; Gloag et al. 2007). Although growth conditions may not yet have been optimised, it is possible that the low growth rate could be related to the novel photochemistry of this cyanobacterium.
Although bacteriochlorophylls in Type II reaction centres of anoxygenic bacteria do not normally generate an oxidant with a sufficiently high potential to remove electrons from water, genetic modification of the reaction centre proteins can yield a photochemical oxidant with a redox potential more positive than +0.8 V, potentially capable of producing oxygen from water (Kálmán et al. 1999; Spiedel et al. 2002; Kálmán et al. 2008). It is known that the photon yield of charge separation in the modified reaction centres is only 5–10% of that of the wild type, and charge recombination is faster (Kálmán et al. 1999, 2008); the modifications to the oxidising side have apparently not altered the redox properties of intermediates on the reducing side of the reaction centre.
For oxygenic organisms as a whole it is important to remember the occurrence of a number of chlorophylls other than chlorophylls a and d. These are chlorophyll b, chlorophylls c and Mg 2,4(or 3,8)divinyl, 2,4(or 3,8)desethyl-phaeoporphyrin a 5 monomethyl ester (Larkum 2003, 2006, 2007; Falkowski and Raven 2007). However, these pigments are photochemically silent, as are the majority of the chlorophyll a and d molecules in any given organism, and function solely in light harvesting and excitation energy transfer (Larkum 2003, 2006, 2007).
Was the first oxygenic organism like Gloeobacter?
Gloeobacter violaceous Rippka, Waterbury et Cohen-Bazire (Nakamura et al. 2003) is the only known cyanobacterium that lacks thylakoids, and molecular phylogenetic analyses show that it is the most basal extant cyanobacterium, that is, it is closest to the ancestral cyanobacterium. What implications for the energetics of photochemistry arise from the occurrence in the plasmalemma of the membrane-associated redox reactions that occur in the thylakoids of all other oxygenic organisms?
The complete genome sequence of G. violaceous shows the absence of several genes associated with the two photosystems, including a number related to the oxygen evolution reactions that occur in the periplasm, and changes to the sequences of periplasmic genes are associated with PSI (plastocyanin: petE) and PSII (e.g. psbO: water oxidation complex) (Nakamura et al. 2003; Mimuro et al. 2008). Functional measurements on PSII suggest less difference between Gloeobacter and cyanobacteria with thylakoids for the oxidising side (facing the periplasm) than for the reducing side (Koyama et al. 2008). For PSI, Gloeobacter uses menaquinone rather than phylloquinone as the secondary electron acceptor. It is not known if these differences impact significantly on overall non-cyclic electron transport and proton pumping.
Regardless of whether the rate or stoichiometry of proton pumping in Gloeobacter is decreased relative to the most comparable thylakoid-containing organisms, the protons are pumped from the cytosol into the periplasm when the protons are in electrochemical equilibrium with those in the bulk medium. The natural medium, and the growth medium, have an alkaline pH, so the pH difference component (Belkin et al. 1987) of the trans-plasma membrane proton electrochemical potential difference (inside negative) is small. This means that most of the driving force for ADP phosphorylation using the CF0–CF1 ATP synthetase must be a cytosol-negative electrical potential difference (Kramer et al. 2003): the trans-plasma membrane electrical potential difference has not been quantified in Gloeobacter, but it is invariably inside-negative in Bacteria (and other organisms) growing in an alkaline medium (Raven 1984a ).
It is of interest that the CF0–CF1 ATP synthetase of Gloeobacter has a 15 : 3 stoichiometry of the proton-conducting c subunits to the adenine nucleotide-binding β subunit, so that the predicted ratio of protons moving from periplasm to cytosol to ADP phosphorylated is 15/3 or 5 (Pogoryelov et al. 2007; Sielaff et al. 2008). The two other cyanobacterial strains with high stoichiometry live in very alkaline media, and so, like Gloeobacter, may have to cope with relatively small proton electrochemical potential differences across the membrane (Pogoryelov et al. 2007). Mesophilic strains have a stoichiometry of 14 : 3, whereas thermophilic strains have 13 : 3 ratios (Pogoryelov et al. 2007). These rationalisations of the ecological occurrence of different c : β stoichiometries in the ATP synthetase must be considered in the context of thermodynamic measurements of the H+ : ATP ratio of 4.0 in the ATP synthetase from both Spinacia chloroplasts (c : β = 14, theoretical H+ : ATP = 4.67) and Escherichia coli plasma membranes (c : β = 10, theoretical H+ : ATP = 3.33) (Steigmiller et al. 2008). Although the thermodynamic measurements are less precise than those from structural biology, to be unable to distinguish between 3.33 and 4.67 seems unlikely, and further attempts at reconciliation between the two techniques are needed.
Could oxygenic photosynthesis occur using photochemistry based on excitation levels equivalent to photon absorption at wavelengths greater than ~700 nm?
On the basis of the energetic arguments used above the answer to this question is ‘no’, at least using two photochemical events to move an electron from water to carbon dioxide. Ever-decreasing photon yields of primary photochemistry, in part as a result of an increased occurrence of back reactions, would increasingly offset any additional energy conversion resulting from the use of a larger fraction of the solar wavelength range reaching the Earth’s surface until, with further increases in wavelength, there would be negligible overall energy gain.
However, oxygenic photosynthesis could occur using excitation levels equivalent to even longer wavelengths if three (or more) photochemical reactions were used in moving an electron from water to carbon dioxide. The seminal paper of Hill and Bendall (1960), which formalised the ‘zig-zag scheme’ or ‘Z scheme’ using two light reactions, included a diagram of a three light reaction scheme, a mechanism that was rejected on the basis of the effect of light on the redox state of cytochromes f and b 6. Using the same fraction of energy from photons to produce the primary reductant and oxidant as discussed above for the two photon scheme would permit a three photon mechanism use of excitation states equivalent to absorption at 1050 nm, and a four photon mechanism would permit extension to 1400 nm. Wavelengths above 1000 nm are perhaps becoming marginal for driving photochemistry (Wolstencroft and Raven 2002; Kiang et al. 2007a , 2007b ), and on Earth 1000 nm is the approximate upper wavelength limit on photochemistry for energy conversion in anoxygenic photosynthetic bacteria (Stomp et al. 2007). These bacteria are free of the constraint of extracting electrons from water and even, in many cases, reducing carbon dioxide because they use photo-heterotrophy and rely in whole or in part on photochemical generation, via electron flow, of transmembrane proton electrochemical potential gradients that can be used in solute transport and ADP phosphorylation (Raven 2009).
The observed upper wavelength limits for biological energy-converting photochemistry on Earth may not apply to possible photosynthetic organisms on planets orbiting stars that are smaller and cooler than the sun and, as a result, have a longer wavelength of maximum emission of electromagnetic radiation. Particular attention has been paid to starts of spectral type ‘M’ (‘Red Dwarfs’), which are longer lived than our sun (Wolstencroft and Raven 2002; Raven and Wolstencroft 2004; Kiang et al. 2007a , 2007b ; Raven 2007), and for which appropriate planetary hosts for life have been found (Beust et al. 2008). Any oxygenic photosynthesis on such planets could involve three (or four) photon mechanisms in moving one electron from water to carbon dioxide, with the consequent lower theoretical lower limit on photon costs of 12 and 16 photons per oxygen produced and carbon dioxide reduced to carbohydrate, rather than the 8 on Earth (Wolstencroft and Raven 2002).
One constraint on the photochemical use of longer wavelengths for photosynthesis is the absorption properties of water, a property that, like thermodynamics, has no known astronomical limits. This has been analysed by, for example, Kirk (1983) and in detail by Stomp et al. (2007), who relate the longer-wavelength absorption bands for photosynthetic pigments and photochemistry to minima in the absorption of radiation by water, as mentioned in an astrobiological context by Wolstencroft and Raven (2002), Raven and Wolstencroft (2004) and Kiang et al. (2007a , 2007b ). Such constraints apply particularly to organisms under increasing depths of liquid water, for example, those photosynthetic bacteria that can only carry out photosynthesis in the absence of oxygen and thus occur below the oxycline in bulk water or in sediments (Raven 2009).
The conclusions from this discussion are that oxygenic photosynthesis could occur at wavelengths in excess of ~700 nm, but would require three or four photons per electron, and would be constrained, particularly in aquatic organisms, by strong absorption by water. Such photosynthesis is not known from Earth.
A final point is that the emphasis on the long wavelength limit for photosynthesis must not blind us to the fact that the conventional lower limit of 400 nm in defining photosynthetically active radiation is not precise. Chlorophyll a absorption extends below 400 nm, and these wavelengths can drive photosynthesis (Halldal 1967), although there is an increasing, dose-dependent inhibition of photosynthesis at decreasing wavelengths in the UV-A (320–400 nm) and nothing, but photodamage in the UV-B range (280–320 nm).
Photon harvesting in oxygenic photosynthetic organisms: filling in the gaps?
Having set the upper wavelength limits for oxygenic photosynthesis on Earth, we can explore the range of pigments that are used in photon harvesting at shorter wavelengths with excitation energy transfer to the photochemically active chlorophyll a (more rarely chlorophyll d). The use of redox silent antenna pigments (in association with protein) to harvest photons in addition to the photochemically active forms of chlorophyll a can be regarded as having three evolutionary rationales. These are increasing the spectral range over which photons are harvested, economising the resources used to construct the photosynthetic apparatus, and restricting the impact of back reactions.
Increasing the spectral range over which photons are harvested can be regarded as ‘filling in the gaps’ in the absorption spectrum of chlorophyll a (Larkum 2003). The addition of redox-silent chlorophyll a to reaction centres as core antenna occurs in both photoreactions, with more pigment molecules per reaction centre for photoreaction I. Additional chlorophyll a in a light-harvesting complex further contributes to decreasing the overall construction costs of the photosynthetic apparatus and to minimising back reactions associated with oxygen production, but only contributes to increasing spectral cover by increasing the absorption of green light, in the trough of absorption by chlorophyll a, at the expense of a significant package effect at the red and, particularly, the blue peaks.
The package effect (Duyens 1956) occurs whenever incident photons at a given wavelength cannot be absorbed by a given chromophore molecule because they have already been absorbed by chromophores that are nearer the photon source. This self-shading obviously sets in first at the absorption maxima of the chromophore, so filling in absorption minima (green for chlorophyll a) by increasing the concentration of the chromophore that is already experiencing a significant package effect at absorption maxima (Terashima et al. 2009) is more costly of resources for synthesis than is adding a different chromophore with absorption maximum complementary to those of the resident chromophore (Kirk 1983). The significantly greater nitrogen and energy costs of making 1 mol of chromophore plus protein in phycoerythrin compared with making 1 mol of chlorophyll a and associated apoprotein are offset by the much greater specific absorption coefficient of phycoerythrin than of chlorophyll a at 550 nm (Raven 1984a ; Alberte 1989; Ting et al. 2002). The costs and benefits of producing light-harvesting pigments are taken up again when chlorophyll-based and retinal-based systems of photochemistry with an energetic output are compared.
The extent to which adding spectrally different pigments to chlorophyll a increases the benefit in terms of light-harvesting capacity relative to the resource costs of producing the additional pigment depends on several factors in addition to variations among pigments in resource cost: the natural radiation field and the optical thickness (extent of package effect) of the organism are two important considerations. The greater the package effect, the smaller the benefit in photon absorption in a given light field of adding another pigment (Dring 1981; Kirk 1983; Lüning 1983; Raven 1984a , 1984b , 1996). Raven (1996) attempted to rationalise the range of photosynthetic pigments in oxygenic organisms in terms of the size range of the clades to which the organism belongs. There is certainly a greater range of pigments in small organisms (thickness less than 10 μm) as a whole than in thicker organisms. However, there is a strong phylogenetic signal here: even including microorganisms in mats or colonies with the larger organisms (red, green and brown algae and embryophytic plants) there are fewer different photosynthetic pigments than when all small organisms are included, but there are also many more clades represented when only small organisms are considered (Raven 1996). Among the small organisms there are clades with very few types of pigment, for example, the Eustigmatophyceae, with essentially only chlorophyll a and violoxanthin.
Turning to the natural radiation field, there is much more diversity in both the range of spectral types of seawater and the vertical zonation of benthic algae than was envisaged when Engelmann (1883) proposed the hypothesis of complementary chromatic adaptation, with green, then brown, then red algae dominating with increasing depth in the inter- and sub-tidal (Dring 1981; Kirk 1983; Lüning 1983). For small organisms there is often a better correlation between pigmentation and the spectral qualities of their natural habitats (Wood 1985; Beard et al. 2000; Stomp et al. 2004, 2007).
Economising on the number of photochemical reaction centres and on the related thylakoid-associated non-photochemical oxidation–reduction, proton pumping and hydration–dehydration catalysts decreases the resource costs of producing the photosynthetic apparatus. The photochemical reaction centres and associated catalysts have some construction costs that can be expressed in the same currency as those of the light-harvesting pigment–protein complexes, that is, nitrogen, carbon and energy (Raven 1984b ). Constructing the reactions centres and associated machinery also has resource costs that are different from those of the antenna pigment–protein complexes, for example, trace metals such as iron, manganese and copper (Raven et al. 1999).
A final rationale for having reaction centres and antennas is that back reactions of photosynthesis, which are essentially independent of the rate of receipt of excitation energy, become increasingly important in photosynthesis at low photon flux densities. One such back reaction is proton leakage through the coupling (thylakoid, except in Gloeobacter) membrane (see Raven et al. 2000). Another, recognised particularly by Radmer and Kok (1977), is that of back reactions in photoreaction II involving intermediates of the oxygen production pathway (see Raven et al. 2000; Quigg et al. 2006). Minimising the number of PSII reaction centres per unit light-harvesting machinery, to an extent compatible with efficient excitation energy transfer to the reaction centres, minimises the pool size of intermediates that could undergo back reactions and hence, with first-order kinetics, the rate of back reaction. Such considerations are clearly of more importance to organisms adapted, or acclimated, to growth at low photon flux densities than to organisms that spend all, or most, of their daylight hours in high photon flux densities when back reactions are relatively less important and dealing with potentially damaging high irradiances may be more significant.
Of these rationales related to the occurrence and diversity of photosynthetic antennae in oxygenic organisms, some are common to anoxygenic photosynthetic organisms, for example, increasing the spectral range of light harvesting and economising in the use of energy, carbon, nitrogen and iron in building the photosynthetic apparatus. Others are specific to oxygenic photosynthesis, that is, the need for manganese and, if the organism cannot make cytochrome c 6, copper, and the back reactions involving the intermediates of oxygen production.
Rhodopsins and retinal-based photochemical energy transformation
Carotenoids have numerous functions in oxygenic photosynthesis, as light-harvesting pigments (e.g. fucoxanthin, peridinin, siphonoxanthin and violoxanthin), as dissipators of excess excitation energy (e.g. diatoxanthin and zeaxanthin), and as quenchers of reactive oxygen species such as singlet oxygen (β-carotene) (Falkowski and Raven 2007). In addition to these functions within the photosynthetic membrane, lipid droplets are thought to screen the photosynthetic apparatus from high-incident irradiances. Also related to photosynthesis is the phototactic response of oxygenic photosynthetic flagellates, enabling them to achieve a position in a radiation gradient, presumably representing a compromise between photosynthesis and photodamage. In green phytoflagellates, such as Chlamydomonas, the light sensor is the β-carotene derivative retinal associated with an opsin polypeptide to form a rhodopsin, and this also seems to be the case for cryptophyte flagellates (Sineshchekov and Govorunova 1999; Sineshchekov et al. 2005; Hegemann 2008). However, until recently it was not known that rhodopsins might also function in energy-transforming photochemistry as well as sensory photochemistry in oxygenic organisms.
Energy-transforming rhodopsins were discovered in the hyperhalophilic archaeon Halobacterium, and have subsequently been demonstrated at the genomic level in other Archaea and Bacteria in marine, estuarine and freshwater habitats (Sharma et al. 2006; McCarren and DeLong 2007; Atamna-Ismaeel et al. 2008; Sharma et al. 2008; Raven 2009). Rhodopsins have a single retinal chromophore associated with the polypeptide opsin; xanthorhodopsin from the bacterium Salinibacter ruber Anton and Oren also has a single carotenoid light-harvesting antenna molecule associated with the opsin (Imasheva et al. 2006). The observed distribution of the energy-transforming rhodopsins in Archaea and Bacteria seems to involve significant horizontal (i.e. lateral) gene transfer (Sharma et al. 2006). These energy-transforming rhodopsins are integral plasma membrane proteins that typically pump protons from the cytosol to the medium, although some pump chloride ions from the medium to the cytosol and one proton pumping rhodopsin has been shown to pump from the medium to the cell (reversed vectoriality) at low external pH (Friedrich et al. 2002). Occasionally there are multiple rhodopsins in a single cell, including representatives of chloride- and proton-pumping rhodopsins as well as sensory rhodopsins of organisms other than metazoans (Bryant and Friggard 2006). Retinal–opsin interactions in proteorhodopsins have genetic variations in the absorption maximum in a way that seems to agree with the transmission properties of the aquatic environment (Sabehi et al. 2007). All of these rhodopsins are Type 1, distinct in molecular phylogenetics from the purely sensory Type II rhodopsins of metazoans (Sharma et al. 2006; McCarren and DeLong 2007).
Archaea and Bacteria with energy-transducing rhodopsins as their sole potentially autotrophic energy conversion mechanism lack autotrophic inorganic carbon assimilation (Raven 2009). This means that the cells cannot grow autotrophically, even if the ion gradient generated by the rhodopsin photochemistry was used to generate NADH from a higher-potential reductant as well as to phosphorylate ADP and power secondary active transport of solutes (Raven 2009). Light stimulation of the growth rate in essentially heterotrophic organisms with energy-transforming rhodopsins is not always observed; when it does occur it appears to occur by allowing more growth per unit organic matter taken up by the organisms. This comes about because less of the organic matter is oxidised to produce ion gradients and thus ATP by oxidative phosphorylation: respiratory generation on these energy-coupling agents is spared by their production from rhodopsin photochemistry (reviewed by Raven 2009). This mode of light energy use in stimulating growth on organic substrates is also found in aerobic anoxygenic photosynthetic bacteria and in some anoxygenic bacteria that only operate photochemistry in anoxia; some members of this latter group are autotrophic. In these cases the photochemical catalyst is a bacteriochlorophyll.
There seem to be no reports of the occurrence of energy-transducing rhodopsins in anoxygenic photosynthetic Bacteria, some of which are autotrophic, or in chemolithotrophic Archaea or Bacteria. This contrasts with the situation for oxygenic photoautotrophs.
Although Type I sensory rhodopsins are well characterised from oxygenic photosynthetic eukaryotes, such as Chlamydomonas reinhardtii P. A. Dangeard, and also occur in some cyanobacteria, until recently energy-transforming rhodopsins had not been characterised from oxygenic phototrophs. Now two such energy-transforming rhodopsins have been characterised, one from the marine ulvophycean green alga Acetabularia acetabulum (L.) P. C. Silva (Tsunoda et al. 2006) and the other from the basal cyanobacterium Gloeobacter violaceous (Miranda et al. 2009).
The Acetabularia opsin cDNA was expressed in Xenopus, where it acted as a proton pump in the plasmalemma, moving (as is typical for these pumps) protons from the cytosol to the medium. This is not easily reconciled with the putative effect of the rhodopsin in Acetabularia as the cause of the rapid green light-dependent depolarisation of the inside-negative transplasmalemma potential produced by the light-dependent chloride influx pump, which involves an influx rather than an efflux of positive charge (Schilde 1968; Gradmann 1978; Tsunoda et al. 2006). Friedrich et al. (2002) showed medium to cytosol pumping of protons by bacteriorhodopsin at an acid external pH value (pH 5.5) when the inside-negative electrical potential was more negative than –80 mV. Although the second criterion is met for Acetabularia (inside ~180 mV more negative than the medium), the seawater medium is at pH ~8 (Schilde 1968; Gradmann 1978; Tsunoda et al. 2006).
The Gloeobacter rhodopsin was heterologously expressed in E. coli to produce enough protein for incorporation into liposomes and characterised spectroscopically (Miranda et al. 2009). These experiments showed that rhodopsin pumps protons from the cytosol to the medium. Gloeobacter has no thylakoids, so all the respiratory and photosynthetic proton pumps, as well as any expression of the rhodopsin proton pump, occur in the plasmalemma. It does not yet seem to be clear (e.g. from ESTs) if the relevant genes are transcribed, let alone expressed, as a functional protein in Gloeobacter.
A proteorhodopsin-like sequence has been found in the marine dinoflagellate Pyrocystis lunula (Schfitt) Schfitt (Okamoto and Hastings 2003; McCarren and DeLong 2007), but has not been functionally investigated.
Comparison of oxygenic photosynthesis with proton-pumping rhodopsins
The occurrence of a rhodopsin-based energy transduction system in oxygenic phototrophs is somewhat surprising. Regardless of its function, it seems that the expression of only two genes is needed for rhodopsin function in organisms that can synthesise β-carotene, that is, a gene encoding an oxidase to produce retinal from β-carotene and a gene encoding the opsin apoprotein (Ruch et al. 2005). This contrasts with the plethora of genes involved in light harvesting, photochemistry, electron transport and proton pumping in oxygenic photosynthesis (Falkowski and Raven 2007).
There is a much greater density per unit membrane area of photochemical reaction centres in an ion-pumping rhodopsin ‘purple membrane’ (~270 nmol m−2; 135 nmol m−2 if half of the membrane is purple membrane, allowing room for F0F1-type ATP synthetases, respiratory enzymes and solute transporters) than in the thylakoid membrane of an oxygenic phototroph (~5 nmol m−2 for each photosystem for a sun-adapted or acclimated vascular plant) (Chapter 2 of Raven 1984b ) (Table 1). A higher density of PSI reaction centres can be calculated for the plasmalemma of Gloeobacter using the chlorophyll per cell for cells grown at high irradiances, the chlorophyll : P700 ratio from Koenig and Schmidt (1995) and the cell dimensions from Holt et al. (1994). This density of 18 nmol m−2 for high light-acclimated cells is not unexpected given the higher PSI : PSII ratio in cyanobacteria and algae with phycobilins as major light-harvesting components than in vascular plants (Raven 1984b ; Falkowski and Raven 2007). However, oxygen flash yield data on Gloeobacter grown at low irradiances (Koyama et al. 2008) suggest 185 chlorophyll a per PSII reaction centre, so the PSI : PSII ratio is only 1.06, and the density of the photosystems in low-light cells is 34 nmol m−2 for PSII and 36 nmol m−2 for PSI.
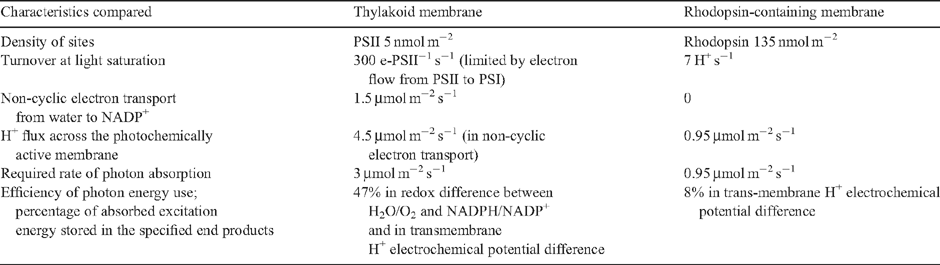
|
The light-saturated specific reaction rate of the overall reaction is ~7 s−1 for the rhodopsin H+ pump (Tsunoda et al. 2006; Miranda et al. 2008) and ~300 s−1 for non-cyclic electron transport with associated proton pumps, expressed in terms of PSII reaction centres (Falkowski and Raven 2007). Multiplying the density of the photochemical reaction centres by the maximum specific reaction rate gives a flux rate of energised product on an area basis. For rhodopsin this is ~0.95 μmol H+ m−2 s−1, whereas for oxygenic photosynthesis it is ~1.5 μmol electron m−2 s−1 moved from water to NADP+ and 4.5 μmol H+ m−2 s−1 (H+ : electron = 3: Falkowski and Raven 2007) (Table 1). Although a higher value could be calculated for the high density of PSII units in the plasmalemma of Gloeobacter, data from Koyama et al. (2008) are consistent with a lower specific reaction rate for overall non-cyclic electron flow in this cyanobacterium than the 300 s−1 used in the estimate above. With an absorbed photon requirement of one photon per H+ with rhodopsins and at least two photons per electron in oxygenic photosynthesis, the fluxes quoted require absorption of ~0.945 μmol photon m−2 s−1 for rhodopsin (each rhodopsin absorbs into one photon) and ~3 μmol photon m−2 s−1 for oxygenic photosynthesis (where most of the photons are absorbed by antenna pigments) at light saturation (Table 1).
The higher capacity to harvest photons at incipient light saturation (Ek ) in oxygenic organisms is paralleled by a higher chromophore density per unit area of membrane. Thus, the kDa protein corresponding to 1 mol of chlorophyll and (light-harvesting) carotenoid in light-harvesting and reaction centre pigment–protein complexes in oxygenic organisms lacking phycobilin antennae is 2.0–6.3, whereas the value for phycobilin antennae is 5.8–15.7 (Raven 1984a , 1984b ; Alberte 1989; Ting et al. 2002). For rhodopsin ion pumps the corresponding value is 26 (Raven 1984b ). This makes rhodopsins much more costly in terms of energy and nitrogen to synthesise than the light-harvesting and photochemical machinery of oxygenic organisms (Raven 1984a , 1984b ; Alberte 1989; Ting et al. 2002). Even when the other electron transfer and proton pump components in oxygenic organisms (e.g. cytochrome b 6–f complex, cytochrome c 6 or plastocyanin, ferredoxin or flavodoxin) are considered (see Raven et al. 1999), the nitrogen and energy costs of rhodopsins are still significantly higher per unit chromophore than the oxygenic organism’s machinery.
If there is the same integral membrane protein content per unit membrane area in oxygenic photosynthesis and in organisms with rhodopsins (see Raven 1984b ), then there is a higher photon absorption rate per membrane area in a given light field for oxygenic organisms. On this basis of comparison, the higher protein per unit chromophore in phycobilin antennae is immaterial because the light-harvesting complexes are extrinsic to the membrane. Other comparisons of chlorophylls and rhodopsins as bioenergetically significant chromophores do not seem to have explicitly addressed the question of the costs of synthesis, but have emphasised functionality (e.g. Bryant and Friggard 2006).
These comparisons of the energy and nitrogen costs in relation to photon absorption and the energised products formed involve several approximations, but the conclusion seems robust: the machinery of oxygenic photosynthesis works faster on a membrane-area basis at light saturation, and has lower energy and nitrogen costs for its synthesis. The costs of synthesis of the oxygenic apparatus are infinitely greater for the trace metals, iron, manganese and (in some cases) copper, required as redox catalysts (Raven et al. 1999) because no trace metals are required in rhodopsins.
Comparing the operating costs of oxygenic photosynthesis, moving one electron from water to NADP+ with the coupled pumping of three protons across the photosynthetic membrane takes at least two absorbed photons given the relatively low photon yield of PSII. The energy stored in the products listed in the last sentence is 110 kJ + (3 × 18 kJ) or 164 kJ per mol electron moved (Raven 1984b ). One mol 680 nm photon (PSII) contributes 176 kJ and one mol 700 nm photon (PS1) contributes 171 kJ, a total of 347 kJ. Even with 100% photochemical efficiency of both photosystems the overall efficiency of energy capture is 164/347 (i.e. 47%) (Table 1). For the mean wavelength of photosynthetically active radiation (i.e. 550 nm), the efficiency is 163/(2 × 218) or 37%. For an ion-pumping rhodopsin with an absorption maximum at 550 nm, the energy input is 218 kJ per mL photon. As the energy stored in the movement of a single mol of protons is only 18 kJ, the efficiency is only 8% (Table 1).
These analyses suggest that there would be very few situations where rhodopsins would be the selectively favoured means of photochemical generation of ion gradients and ATP. Iron deficiency could be one situation; cyclic electron flow in photosynthesis is very iron intensive, so in low-iron environments it might be favoured. Could the even more iron-requiring process of diazotrophy be such a case? There seem to be no reports of energy-transforming rhodopsins in marine cyanobacterial diazotrophs, such as the completely sequenced Crocosphaera and Trichodesmium: is this also the case for UCYN-A, a photoorganotrophic diazotrophic organism lacking PSII and Rubisco (Zehr et al. 2008), and which presumably functions physiologically like aerobic anoxygenic photosynthetic bacteria (Raven 2009). Another possibility where energy-transforming rhodopsins could offer a competitive advantage is environments where green light predominates: here such advantages are greatest in optically thin organisms with a small package effect, and even in this case phycobilins can fill in the ‘green gap’ in most cyanobacteria, glaucocystophytes and red algae.
Conclusions
Some of the properties of chlorophyll a can be rationalised in terms of the energetics of oxygenic photosynthesis with two photochemical reactions and the solar spectrum. The same is true of some aspects of the diversity of light-harvesting pigments, and the properties of energy-transforming rhodopsins and their restricted global role relative to that of the chlorophylls. However, rationalising is not the same as showing that the factors considered were important in the evolution of what we see around us.
Acknowledgements
Discussions with John Beardall, Charles Cockell, Paul Falkowski, Kevin Flynn, Richard Geider, Mario Giordano and Tony Larkum were very helpful. This paper was significantly improved by comments on an earlier version from Tony Larkum and from two anonymous reviewers. The University of Dundee is a registered Scottish charity (No: SC015096).
Allen JF
(2003) Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends in Plant Science 8, 15–19.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Allen JF
(2004) Cytochrome b6f: structure for signalling and vectorial metabolism. Trends in Plant Science 9, 130–137.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Allen JF
(2005) A redox switch hypothesis for the origin of two light reactions in photosynthesis. FEBS Letters 579, 963–968.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Allen JF, Martin W
(2007) Out of thin air. Nature 445, 610–612.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Atamna-Ismaeel N,
Sabehi G,
Sharon I,
Wizel K-P,
Labrenz M,
Jürgens K,
Barkay T,
Stomp M,
Huisman J, Beja O
(2008) Widespread distribution of proteorhodopsins in freshwater and brackish ecosystems. The ISME Journal 2, 656–662.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Beard SJ,
Davis PA,
Iglesias-Rodríguez D,
Skulberg OM, Walsby AE
(2000) Gas vesicle genes in Planktothrix spp. from Nordic lakes: strains with weak gas vesicles possess a larger variant of gupC. Microbiology (Reading, England) 146, 2009–2018.
|
CAS |
PubMed |

Belkin S,
Mehlhorn RJ, Packer L
(1987) Proton gradients in intact cyanobacteria. Plant Physiology 84, 25–30.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Beust H,
Bonfils X,
Delfosse X, Udry S
(2008) Dynamical evolution of the Gliese 581 planetary system. Astronomy & Astrophysics 479, 277–282.
| Crossref | GoogleScholarGoogle Scholar |

Björn LO,
Papageorgiou GC,
Blankenship RE, Govindjee
(2009) A viewpoint: why chlorophyll a? Photosynthesis Research 99, 85–98.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bryant DA, Friggard N-U
(2006) Prokaryotic photosynthesis and phototrophy illuminated. Trends in Microbiology 14, 488–496.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Clausen J, Junge W
(2008a) The terminal reaction cascade of water oxidation: proton and oxygen release. Biochimica et Biophysica Acta 1777, 1311–1318.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Clausen J, Junge W
(2008b) The inhibitory effects of acidification and augmentated oxygen pressure on water oxidation. Photosynthesis Research 98, 229–233.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Clausen J,
Junge W,
Dau H, Haumann M
(2005) Photosynthetic water oxidation at high O2 backpressure monitored by delayed chlorophyll fluorescence. Biochemistry 44, 12775–12779.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Cser K,
Deák Z,
Telfer A,
Barbe J, Vass I
(2008) Energetics of photosystem II charge recombination in Acaryochloris marina studied by thermoluminescence and flash-induced chlorophyll fluorescence measurements. Photosynthesis Research 98, 131–140.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Dring MJ
(1981) Chromatic adaptation of photosynthesis in benthic algae: an examination of its ecological significance using a theoretical model. Limnology and Oceanography 26, 271–284.

Duyens LNM
(1956) The flattening of the absorption of suspensions, as compared to that of solutions. Biochimica et Biophysica Acta 19, 1–12.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Engelmann TW
(1883) Farbe und assimilation. Botanische Zeitung 41, 1–29.

Friedrich T,
Geibel S,
Kalmbach R,
Chizhov I,
Ataka K,
Heberle J,
Engelhard M, Bamberg E
(2002) Proteorhodopsin is a light-driven proton pump with variable vectoriality. Journal of Molecular Biology 321, 821–838.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Gloag RS,
Ritchie RJ,
Chen M,
Larkum AWD, Quinnell RG
(2007) Chromatic photoacclimation, photosynthetic electron transport and oxygen evolution in the chlorophyll d-containing oxyphotobacterium Acaryochloris marina. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1767, 127–135.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Gomez A,
Chew M, Bryant DA
(2007) Chlorophyll biosynthesis in bacteria: the origins of structural and functional diversity. Annual Review of Microbiology 61, 113–129.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gradmann D
(1978) Green light (550 nm) inhibits electrogenic Cl− pump in Acetabularia membrane by permeability increase for the carrier ion. The Journal of Membrane Biology 44, 1–24.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Halldal P
(1967) Ultraviolet action spectra in algology. A review. Photochemistry and Photobiology 6, 445–460.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hancke TB,
Hancke K,
Johnsen G, Sakshaug E
(2008) Rate of O2 production derived from pulse-amplitude-modulated fluorescence: testing three biooptical approaches against measured O2-production rate. Journal of Phycology 44, 803–813.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Haumann M,
Grundmeier A,
Zaharieva I, Dau H
(2008) Photosynthetic water oxidation at elevated dioxygen partial pressure monitored by time-resolved X-ray absorption measurements. Proceedings of the National Academy of Sciences of the United States of America 105, 17 384–17 389.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hegemann P
(2008) Algal sensory photoreceptors. Annual Review of Plant Biology 59, 167–189.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hill R, Bendall F
(1960) Function of two cytochrome components in chloroplasts: a working hypothesis. Nature 186, 136–137.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Imasheva ES,
Balashov SP,
Wang JM, Lanyi JK
(2006) pH-dependent transitions in xanthorhodopsin. Photochemistry and Photobiology 82, 1406–1413.
|
CAS |
PubMed |

Johnsen G, Sakshaug E
(2007) Biooptical characteristics of PSII and PSI in 33 species (13 pigment groups) of marine phytoplankton, and the relevance for pulse-amplitude-modulated and fast-repetition-rate fluorometry. Journal of Phycology 43, 1236–1251.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Johnson G
(2004) Controversy remains: regulation of pH gradient across the thylakoid membrane. Trends in Plant Science 9, 570–571.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Kálmán L,
LoBrutto R,
Allen JP, Williams JC
(1999) Modified reaction centres oxidize tyrosine in reactions that mirror photosystem II. Nature 402, 696–699.
| Crossref | GoogleScholarGoogle Scholar |

Kálmán L,
Williams JC, Allen JP
(2008) Comparison of bacterial reaction centers and photosystem II. Photosynthesis Research 98, 643–655.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kiang NY,
Siefert J,
Govindjee
, Blankenship RE
(2007a) Spectral signatures of photosynthesis. I. Review of Earth organisms. Astrobiology 7, 222–251.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Kiang NY,
Segura A,
Tinetti G,
Govindjee
,
Blankenship RE,
Cohen M,
Siefert J,
Crisp D, Meadows VS
(2007b) Spectral signatures of photosynthesis. II. Coevolution with other stars and the atmosphere on extrasolar worlds. Astrobiology 7, 252–274.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Koenig F, Schmidt M
(1995)
Gloebacter violaceous – investigation of an unusual photosynthetic apparatus. Absence of the long wavelength emission of photosystem I in 77 K fluorescence spectra. Physiologia Plantarum 94, 621–628.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Kolling DRJ,
Brown TS,
Ananyev G, Dismukes GC
(2009) Photosynthetic oxygen evolution is not reversed by high oxygen pressures: mechanistic consequences for the water-oxidising complex. Biochemistry 48, 1381–1389.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Koyama K,
Suzuki H,
Noguchi T,
Akimoto S,
Tsuchiya T, Mimuro M
(2008) Oxygen evolution in the thylakoid-lacking cyanobacterium Gloeobacter violaceous PCC 7421. Biochimica et Biophysica Acta 1777, 369–378.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Kramer DM,
Cruz JA, Kanazawa A
(2003) Balancing the central roles of the thylakoid proton gradient. Trends in Plant Science 8, 27–32.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Kramer DM,
Avenson TJ, Edwards GE
(2004) Response to Johnson: controversy remains: regulation of pH gradient across the thylakoid membrane. Trends in Plant Science 9, 571–572.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Larkum AWD, Kuhl M
(2005) Chlorophyll d: the puzzle resolved. Trends in Plant Science 10, 355–357.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

McCarren J, DeLong EF
(2007) Proteorhodopsin photosystem gene clusters exhibit co-evolutionary trends and shared ancestry among diverse marine microbial phyla. Environmental Microbiology 9, 846–858.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Mimuro M,
Tomo T, Tsuchiya T
(2008) Two unique cyanobacteria lead to a traceable approach of the first appearance of oxygenic photosynthesis. Photosynthesis Research 97, 167–176.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Miranda MRM,
Choi AR,
Bezerra AH,
Jung K-H, Brown LS
(2009) The photocycle and proton translocation pathway in a cyanobacterial ion-pumping rhodopsin. Biophysical Journal 96, 1471–1481.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mulkidjanian AY, Junge W
(1997) On the origin of photosynthesis as inferred from sequence analysis. A primordial UV-protector as common ancestor of reaction centers and antenna proteins. Photosynthesis Research 51, 27–42.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Nakamura Y,
Kaneko T,
Sato S,
Mimuro M, Miyashita H ,
et al
.
(2003) Complete genome structure of Gloeobacter violaceous PCC 7421, a cyanobacterium that lacks thylakoids. DNA Research 10, 137–145.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ohashi S,
Miyashita H,
Okusa N,
Iemura T,
Watanabe T, Kobayashi M
(2008) Unique photosystems in Acaryochloris marina. Photosynthesis Research 98, 141–149.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Okamoto OK, Hastings JW
(2003) Novel dinoflagellate clock-related genes identified through microarray analysis. Journal of Phycology 39, 519–526.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Pogoryelov D,
Reichen C,
Klyszejko AJ,
Brunisholz R,
Muller DJ,
Dimroth P, Meier T
(2007) The oligomeric state of c rings from the cyanobacterial F-ATP synthases varies from 13 to 15. Journal of Bacteriology 189, 5895–5902.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Quigg A,
Kevekordes K,
Raven JA, Beardall J
(2006) Limitations on microalgal growth at very low photon fluence rates: the role of energy slippage. Photosynthesis Research 88, 299–310.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Radmer R, Kok B
(1977) Photosynthesis – limited yields, unlimited dreams. American Scientist 27, 599–605.

Raven JA
(1984a) A cost-benefit analysis of photon absorption by photosynthetic unicells. New Phytologist 98, 593–625.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Raven JA
(1996) The bigger the fewer: size, taxonomic diversity and the range of pigments in marine phototrophs. Journal of the Marine Biological Association of the United Kingdom 76, 211–217.
| Crossref |

Raven JA
(2007) Photosynthesis in watercolours. Nature 448, 418.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Raven JA
(2009) Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquatic Microbial Ecology in press ,

Raven JA, Larkum AWD
(2007) Are there ecological implications for proposed energetic restriction on oxygen evolution at high oxygen concentrations? Photosynthesis Research 94, 31–42.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Raven JA, Wolstencroft RD
(2004) Constraints on photosynthesis on Earth and Earth-like planets. Bioastronomy 2002: Life Among the Stars 213, 305–308.
|
CAS |

Raven JA,
Evans MCW, Korb RE
(1999) The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynthesis Research 60, 111–149.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Raven JA,
Kübler JE, Beardall J
(2000) Put out the light, and then put out the light. Journal of the Marine Biological Association of the United Kingdom 80, 1–25.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Raven JA,
Cockell CS, De La Rocha CL
(2008) The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363, 2641–2650.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ruch S,
Beyer P,
Ernst H, Al-Babili S
(2005) Retinal synthesis in Eubacteria: in vitro characterization of a novel carotenoid oxygenase from Synechocystis sp. PCC 6803. Molecular Microbiology 55, 1015–1024.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sabehi G,
Kirkup BC,
Rozenberg M,
Stambler N,
Polz MF, Béjà O
(2007) Adapation and spectral tuning in divergent marine proteorhodopsins from the eastern Mediterranean and the Sargasso Seas. The ISME Journal 1, 48–55.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Schilde C
(1968) Rapid photoelectric effect in the alga Acetabularia. Zeitschrift für Naturforschung B 23, 1369–1376.
|
CAS |

Sharma AK,
Spudich JL, Doolittle WF
(2006) Microbial rhodopsins: functional versatility and genetic mobility. Trends in Microbiology 14, 463–469.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sharma AK,
Zhaxybayeva O,
Papke RT, Doolittle WF
(2008) Actinorhodopsins: proteorhodopsin-like gene sequences found predominantly in non-marine environments. Environmental Microbiology 10, 1039–1056.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sielaff M,
Rennekamp H,
Wächter A,
Xie H,
Hilbers F,
Feldbauer K,
Dunn SD,
Engelbrecht S, Junge W
(2008) Domain compliance and elastic power transmission in rotary F0F1-ATPase. Proceedings of the National Academy of Sciences of the United States of America 105, 17 760–17 765.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sineshchekov OA, Govorunova EV
(1999) Rhodopsin-mediated photosensing in green flagellated algae. Trends in Plant Science 4, 58–63.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sineshchekov OA,
Govorunova EG,
Jung K-H,
Zaurer S,
Maier UG, Spudich JL
(2005) Rhodopsin-mediated photoreception in cryptophyte flagellates. Biophysical Journal 89, 4310–4319.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Spiedel D,
Jones MR, Robert B
(2002) Tuning of the redox potential of the primary electron donor in reaction centres of purple bacteria: effects of amino acid polarity and position. FEBS Letters 527, 171–175.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Steigmiller S,
Turina P, Gräber P
(2008) The thermodynamic H+/ATP ratios of the H+-ATP synthetase from chloroplasts and Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 105, 3745–3750.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Stomp M,
Huisman J,
de Jongh F,
Veraart AJ,
Gerla D,
Rijkeboer M,
Iberlings BW,
Wollenzien UIA, Stal LJ
(2004) Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 432, 104–107.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Stomp M,
Huisman J,
Stal LJ, Matthijs HCP
(2007) Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. The ISME Journal 1, 271–282.
|
CAS |
PubMed |

Strzepek RF, Harrison PJ
(2004) Photosynthetic architecture differs in coastal and oceanic diatoms. Nature 431, 689–692.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Swingley WD,
Hohomann-Marriott MF,
Olson TL, Blankenship RE
(2005) Effect of iron on growth and ultrastructure of Acaryochloris marina. Applied and Environmental Microbiology 71, 8606–8610.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Terashima I,
Fujita T,
Inoue T,
Chow WS, Oguchi R
(2009) Green light drives leaf photosynthesis more efficiently than red light in strong white light: revisiting the enigmatic question of why leaves are green. Plant & Cell Physiology 50, 684–697.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ting CS,
Rocap G,
King J, Chisholm SW
(2002) Cyanobacterial photosynthesis in the oceans: the origins and significance of divergent light-harvesting strategies. Trends in Microbiology 10, 134–142.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Tsunoda SP,
Ewers D,
Gazzarrini S,
Motoni A,
Gradmann D, Hegemann P
(2006) H+-pumping rhodopsin from the marine alga Acetabularia. Biophysical Journal 91, 1471–1479.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Wolstencroft D, Raven JA
(2002) Photosynthesis: likelihood of occurrence and possibility of detection in Earth-like planets. Icarus 157, 535–548.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Wood AM
(1985) Adaptation of the photosynthetic apparatus of marine phytoplankton to natural light fields. Nature 316, 253–255.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Zehr JP,
Bench SR,
Crater BJ,
Hewson I,
Niazi F,
Shi T,
Tripp HJ, Affourtit JP
(2008) Globally distributed uncultivated oceanic N2-fixing cyanobacteria lack oxygenic photosystem II. Science 322, 1110–1112.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |



