The contrasting influence of short-term hypoxia on the hydraulic properties of cells and roots of wheat and lupin
Helen Bramley A B D E , Neil C. Turner C D , David W. Turner B and Stephen D. Tyerman AA School of Agriculture, Food and Wine, The University of Adelaide (Waite Campus), Plant Research Centre, PMB 1, Glen Osmond, SA 5064, Australia.
B School of Plant Biology, M084, Faculty of Natural and Agricultural Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
C Centre for Legumes in Mediterranean Agriculture, M080, Faculty of Natural and Agricultural Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
D Institute of Agriculture, M082, Faculty of Natural and Agricultural Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
E Corresponding author. Email: helen.bramley@uwa.edu.au
Functional Plant Biology 37(3) 183-193 https://doi.org/10.1071/FP09172
Submitted: 10 July 2009 Accepted: 18 December 2009 Published: 25 February 2010
Abstract
Little is known about water flow across intact root cells and roots in response to hypoxia. Responses may be rapid if regulated by aquaporin activity, but only if water crosses membranes. We measured the transport properties of roots and cortical cells of three important crop species in response to hypoxia (0.05 mol O2 m–3): wheat (Triticum aestivum L.), narrow-leafed lupin (Lupinus angustifolius L.) and yellow lupin (Lupinus luteus L.). Hypoxia influenced solute transport within minutes of exposure as indicated by increases in root pressure (Pr) and decreases in turgor pressure (Pc), but these effects were only significant in lupins. Re-aeration returned Pr to original levels in yellow lupin, but in narrow-leafed lupin, Pr declined to zero or lower values without recovery even when re-aerated. Hypoxia inhibited hydraulic conductivity of root cortical cells (Lpc) in all three species, but only inhibited hydraulic conductivity of roots (Lpr) in wheat, indicating different pathways for radial water flow across lupin and wheat roots. The inhibition of Lpr of wheat depended on the length of the root, and inhibition of Lpc in the endodermis could account for the changes in Lpr. During re-aeration, aquaporin activity increased in wheat roots causing an overshoot in Lpr. The results of this study demonstrate that the roots of these species not only vary in hydraulic properties but also vary in their sensitivity to the same external O2 concentration.
Additional keywords: hydraulic conductivity, oxygen deficiency, pressure probe, root pressure, turgor pressure.
Introduction
Research investigating the tolerance of different species to waterlogging has predominantly focussed on the long-term effects on growth (days to weeks), yet many physiological processes are affected within much shorter time frames. Waterlogging leads to a depletion of oxygen in the rhizosphere and whilst this depletion can happen quickly, no plant experiences anoxia (zero O2) immediately upon its root system becoming submerged (Blackwell 1983). Examining responses that occur when a plant senses the changing environment will help reveal mechanisms involved in acclimation to O2 deficiency. For example, pre-exposure to hypoxia (low O2) increases the tolerance of a tissue to subsequent anoxia through improved cytosolic pH regulation and respiration, higher rates of alcoholic fermentation and changes in protein production and gene expression (Saglio et al. 1988; Waters et al. 1991; Xia and Roberts 1996; Dennis et al. 2000; Kato-Noguchi 2000). Pre-exposure to hypoxia is also required to stimulate development of lysigenous aerenchyma (Drew et al. 2000).
Waterlogging and low O2 in the rhizosphere tend to reduce the hydraulic conductivity of roots (Lpr), but responses vary between species and type of treatment imposed (see review in Bramley et al. 2007a). Root death or anatomical changes may reduce Lpr during long-term exposure to O2 deficiency by creating physical barriers to water flow. Anatomical changes that influence Lpr are usually associated with water and nutrient deficiencies and have rarely been characterised for waterlogging (Enstone and Peterson 2005). Even less is known about responses in Lpr to short-term exposure to O2 deficiency, but Lpr can change rapidly in response to transpiration and other abiotic perturbations (Steudle 2001). Rapid changes in Lpr due to O2 deficiency may be caused, indirectly or directly, by reduced respiration rates or pH-induced gating of aquaporins (Tournaire-Roux et al. 2003). However, these effects require bulk water flow to cross membranes somewhere along its flow-path between the root surface and vascular tissue. If water flows entirely through the apoplast (cell walls and intercellular spaces), then aquaporins will have little influence on Lpr.
This study examined the effects of short-term (0.5 h) hypoxia on water flow in wheat (Triticum aestivum L.), narrow-leafed lupin (Lupinus angustifolius L.) and yellow lupin (Lupinus luteus L.) roots when all three species were exposed to the same external oxygen concentration (0.05 mol O2 m–3). Wheat and lupins are important crops grown in winter in southern Australia. When sown on duplex soils, where a sandy/loam soil overlies clay, these crops often experience transient waterlogging during periods of high rainfall (Belford et al. 1992). Wheat is thought to tolerate waterlogging better than lupins, although direct comparisons under the same field conditions have not been undertaken (Dracup et al. 1992). In a glasshouse experiment comparing root growth in root chambers flooded for 1 week, wheat roots had superior survival and recovery than narrow-leafed lupin or yellow lupin (Bramley 2006). Roots of both lupin species died when submerged and only yellow lupin grew new roots when the chambers were drained. In a study on whole plants, Davies et al. (2000a) found that yellow lupin was more tolerant to waterlogging than narrow-leafed lupin due to properties of their root system, as indicated by cross-grafting roots and shoots, although it is not yet known what properties confer that response.
The effect of O2 deficiency on water flow in lupin roots has not been previously investigated, but Zhang and Tyerman (1991) found that 0.5 h of hypoxia reduced the hydraulic conductivity of root cortical cells (Lpc) of wheat by 85%. Water uptake in wheat roots occurs predominantly in a region close to the root tip and under ambient conditions, radial water flow is influenced by aquaporins, probably located in the endodermis (Bramley et al. 2009). Therefore, hypoxia may inhibit Lpr of wheat roots and the extent of the inhibition should be related to the inhibition at the cell level. In comparison, radial water flow in lupin roots occurs predominantly through the apoplast, despite significant aquaporin activity in cortical cells (Bramley et al. 2009). Any inhibitory effects of O2 deficiency on aquaporin activity should therefore have less influence on Lpr, unless O2 deficiency causes water flow to switch to the cell-to-cell pathway.
Water flow was measured across individual roots before, during and after a mild hypoxic treatment. Because wheat roots resume growth after waterlogging (Bramley 2006), water uptake must also resume and therefore, Lpr should recover when wheat roots have sufficient oxygen. To determine whether effects on Lpr were due to transport across cell membranes, the effects of hypoxia on Lpc of root cortical cells were also measured. In comparison to the majority of studies that simulate waterlogging or O2 deficient conditions by imposing sudden anoxia (zero oxygen), 0.05 mol O2 m–3 is a mild hypoxic treatment. However, it is similar to the concentration that reduced Lpc of wheat root cells (Zhang and Tyerman 1991) through closure of aquaporins (Zhang and Tyerman 1999). This O2 concentration can also induce changes in aquaporin expression within 0.5 h of treatment (Klok et al. 2002).
Materials and methods
Plant material
Seeds of wheat (Triticum aestivum L. cv. Kulin), narrow-leafed lupin (Lupinus angustifolius L. cv. Merrit) and yellow lupin (L. luteus L. cv. Wodjil) were germinated and grown as described by Bramley et al. (2007b). Plants were grown in individual sand-filled pots that allowed access to the roots without injury. Roots were carefully washed from the sand 10–14 days after sowing. The taproot of lupin or the longest seminal root of wheat was excised below emerging lateral roots as lateral roots prevented the root sealing to the root pressure probe. Roots were 70–180 mm long for measurements with the root pressure probe and roots were excised 80 mm from the tip for measurements on intact cells. Root diameters in the region punctured with the cell pressure probe were 0.61 ± 0.02 mm for wheat, 0.89 ± 0.05 mm for yellow lupin and 1.2 ± 0.20 mm for narrow-leafed lupin.
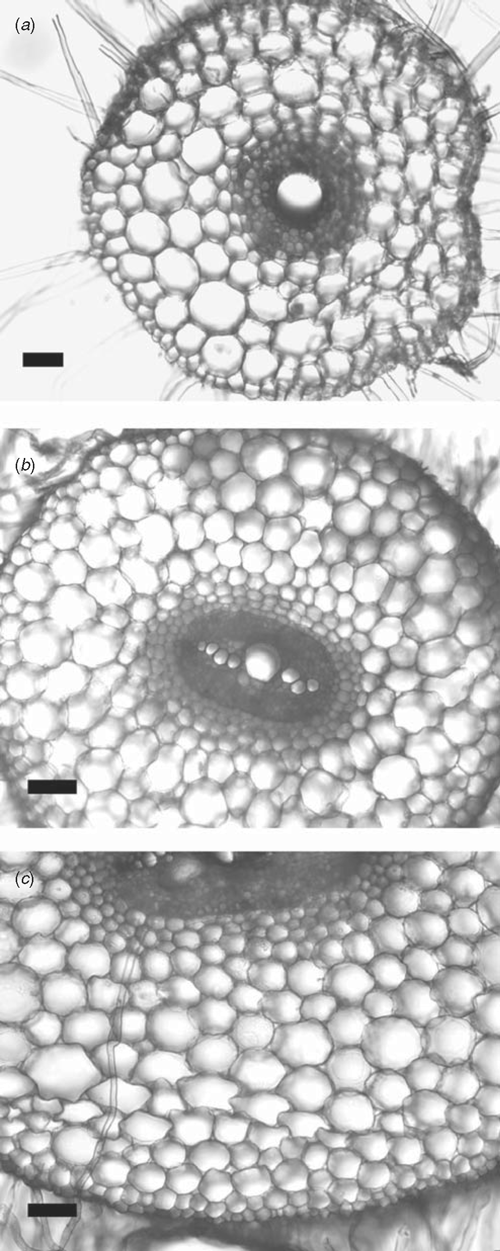
Roots had the same anatomy as those reported in Bramley et al. (2009), with none of the species developing an exodermis and the endodermis matured closer to the root tip in wheat than in either lupin species (Fig. 1).
|
Hydraulic conductivity of roots (Lpr)
The excised end of the root was connected to a root pressure probe (Steudle 1993) via a seal made from silicon impression material (Exaflex, Halas Dental Supplies, Adelaide, SA, Australia). The root was supported inside a glass tube (5 mm internal diameter), which was connected to a reservoir (maintained at 25°C by a water bath) of the same nutrient solution used to water the plants, supplemented with 5 mM glucose. Glucose was included as a surrogate carbohydrate (Gibbs et al. 1998a) supply because measurements on excised roots took several hours. The solution circulated past the root at a rate of 10–15 mm s–1. The nutrient solution was bubbled with air or a combination of N2 plus air (hypoxic treatment), through an air-stone, whilst a Rank oxygen electrode continuously monitored the O2 concentration just before the solution passed the root. When the nutrient solution was bubbled with N2 plus air, it took 120–600 s for the O2 concentration to decrease to 0.05 mol O2 m–3. The circulation system was totally sealed apart from where the root entered the glass tube.
Measurements of Lpr commenced when root pressure (Pr) was stable, which took a minimum of 2 h depending on the individual root. Water flow through the root was induced by applying a series of successive step changes in root pressure by rotating the rod inside the probe (Bramley et al. 2007b). Pressure was clamped for 60–120 s to ensure that pressures and flows were measured under steady-state conditions (Bramley et al. 2007b). The hydraulic conductance of the root (Lr) was calculated from the slope of the linear regression of volume flow rate against applied pressure. Lr was normalised by the surface area of the root to give Lpr. Lpr was measured on the same root three times; during perfusion with aerated solution (0.25 mol O2 m–3), after 0.5 h of hypoxia (0.05 mol O2 m–3) and after 1 h of re-aeration (0.25 mol O2 m–3).
The seal connecting the root to the pressure probe was tested at the end of the measurements to ensure that it had not restricted water flow during the experiment (Steudle 1993).
Water relations of root cortical cells
Effects of hypoxia on cell water relations were determined using a cell pressure probe mounted on a micromanipulator with 1-µm increments (MX1, Narishige, Tokyo, Japan). The root segment was secured inside a small perspex chamber, as described by Zhang and Tyerman (1991), and positioned on the stage of a microscope. A transparent cover was sealed to the top of the chamber with vacuum grease so that the circulatory system was entirely closed, apart from a 5-mm opening for entry of the microcapillary of the pressure probe. The same nutrient solution as that used in measurements of Lpr was circulated through the chamber at a rate of 420 mL h–1 using a peristaltic pump (Exatech, Melbourne, Vic, Australia). The O2 concentration of the nutrient solution was adjusted and monitored as described above.
Cortical cells were punctured by the microcapillary of the pressure probe in the second to sixth cell layer from the root surface (20–200 µm depth from root surface), 30–50 mm from the root tip. The methodology for measuring the water relations of cortical cells and the size of the cells were reported in Bramley et al. (2009). Turgor pressure (Pc), half-time of the rate of water exchange (T1/2) and volumetric elastic modulus (ॉ) were measured and used to calculate the hydraulic conductivity of cells (Lpc). Where water flow across membranes was rapid (T1/2 < 1 s), ॉ was corrected for the curvilinear relationship between volume and pressure change (Steudle et al. 1980). Because of the difficulty in maintaining constant turgor pressure and an unblocked probe capillary for long periods of time, measurements were conducted on roots within 0.2–0.5 h, after bathing roots for 0.5 h with aerated solution (0.25 mol O2 m–3) or hypoxic solution (0.05 mol O2 m–3).
Statistical analyses
All data were analysed with SPSS ver. 11.0 (SPSS, Chicago, IL, USA) and GraphPad Prism ver. 5.01 (GraphPad Software, La Jolla, CA, USA). One-way ANOVA compared the initial root pressure (Pr) between species. To test whether hypoxia significantly changed Pr of each species, Pr before and during hypoxia were compared in a paired t-test. Repeated-measures ANOVA tested the effects of the O2 treatments on Lpr with Bonferroni post-test. Two-way ANOVA with Bonferroni post-tests compared the effects of O2 treatment on the water transport parameters of cells.
Results
Root pressure
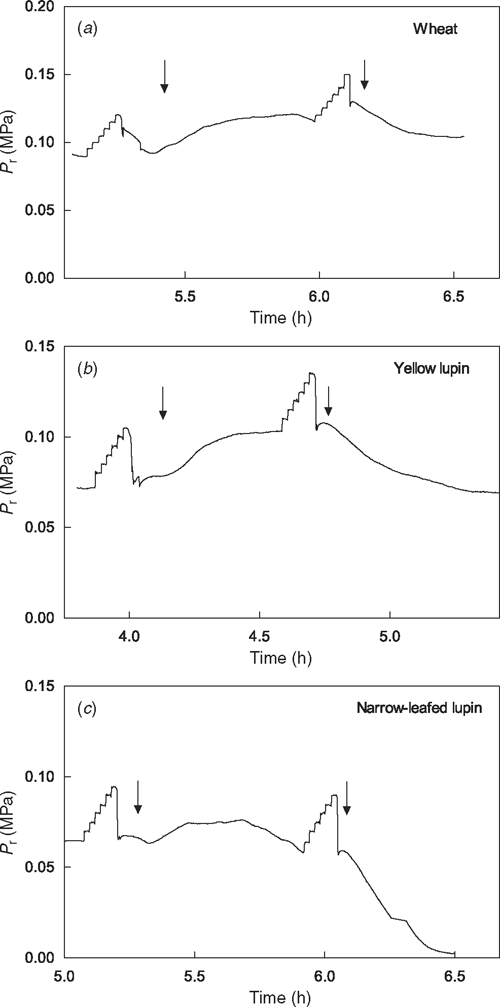
When connected to the root pressure probe, wheat roots generated significantly higher Pr than either species of lupin (P < 0.0001; Table 1). Roots responded to reduced O2 concentration by increased Pr (Table 1, Fig. 2). Pr of both lupin species significantly increased in response to hypoxia (P = 0.001 for narrow-leafed lupin and P = 0.021 for yellow lupin), but the response in wheat was not statistically significant (P = 0.295). Pr of yellow lupin began to increase soon after the commencement of bubbling with N2 plus air when the O2 concentration of the solution was between 0.06 and 0.07 mol O2 m–3, and then returned to pre-hypoxia values when roots were re-aerated (Fig. 2b). In comparison, Pr of narrow-leafed lupin took 161 (±32) s at 0.05 mol O2 m–3 before increasing, but the increase in Pr was only transient, declining during the hypoxic treatment (Fig. 2c). Re-aeration did not arrest the decline in 6 of the 11 narrow-leafed lupin roots, but in those roots, Pr continued to decline to zero without subsequent recovery (Fig. 2c).

|
|
Hydraulic conductivity of roots
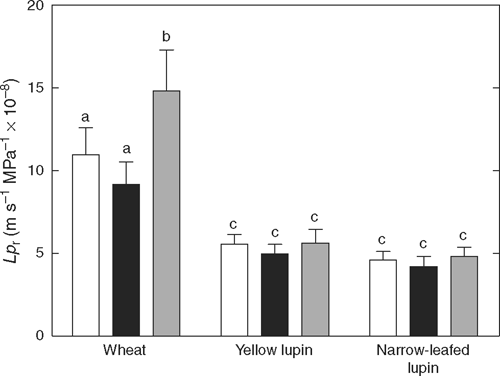
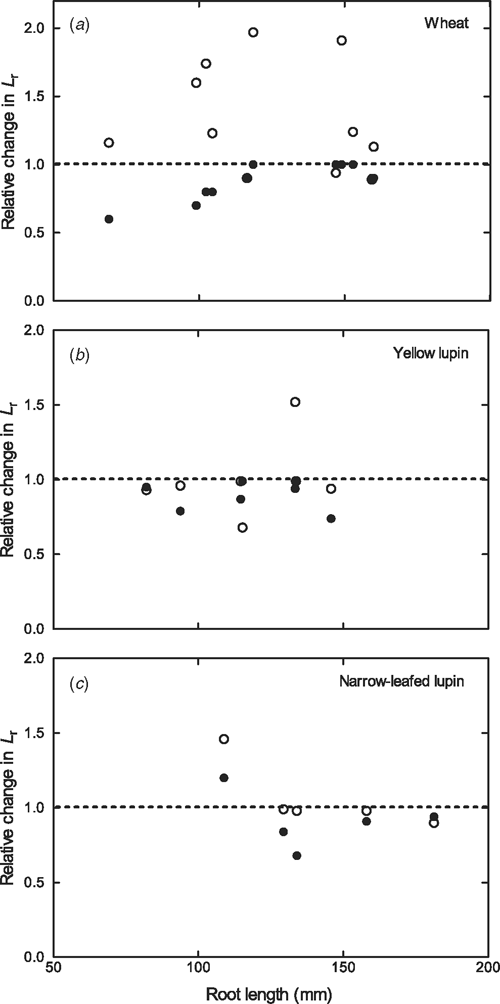
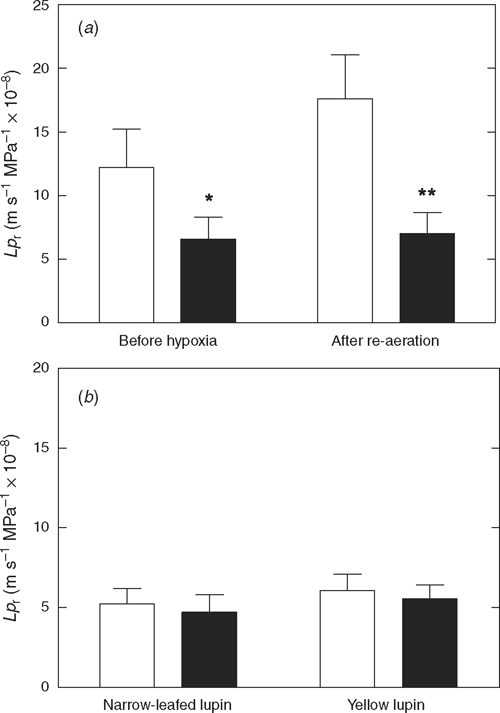
Lpr of aerated wheat roots was almost twice that of both lupin species (Fig. 3; P = 0.008). Aeration treatment significantly affected Lpr of wheat roots (P = 0.0007; Fig. 3), but did not significantly affect Lpr of either lupin species when roots with stable root pressure at the end of the experiment were analysed (P = 0.294 for narrow-leafed lupin and 0.331 for yellow lupin; Fig. 3). The effect of O2 treatment on Lpr was not analysed in lupin roots where Pr decreased to zero without recovery, as this ‘leakiness’ creates artificially high water flows. For wheat roots, hypoxia reduced Lpr by 2–45%, but due to this variability, the effect was not significant (P > 0.05; Fig. 3). However, after re-aeration, Lpr was on average 1.4 and 1.6 times greater than Lpr before and during hypoxia, respectively (P < 0.05; Fig. 3). Because Lpr of individual wheat roots is inversely related to root length (Bramley et al. 2009), the relative change in Lr (Lpr × A) due to O2 treatment (normalised to the value before hypoxia) was examined in relation to root length (Fig. 4). The relative change in Lr due to hypoxia tended to be greater in shorter roots (Fig. 4a), but was independent of root length after re-aeration (Fig. 4a).
|
|
Because changes in root anatomy could be discounted during such short-term aeration treatments, changes in Lpr of wheat roots may have been mediated by varying aquaporin activity. Aquaporin activity was tested before hypoxia and after re-aeration using mercuric chloride to inhibit water flow across cell membranes, the assumption being that if more aquaporins were active there would be greater inhibition of water flow. The influence of aquaporins on Lpr was not tested during hypoxic treatment as the combined treatment of mercury and hypoxia was toxic to roots, causing loss of root pressure without recovery. Wheat roots were incubated for 0.5 h with 50 μM HgCl2 before hypoxia or after 0.5 h hypoxia plus 1 h re-aeration. Aquaporin activity was also tested in lupin roots, but only for pre-hypoxia treatment as Lpr was not significantly affected by O2 treatments.
Mercuric chloride inhibited water flow of wheat roots more after re-aeration (60%) than before hypoxia (40%), reducing Lpr to the same level (Fig. 5a). In comparison, mercury did not significantly affect Lpr of either lupin species (P > 0.05; Fig. 5b).
|
Cell water relations
Cortical cells of wheat had significantly higher turgor pressure than cells of either lupin species (Table 2; P < 0.001). Hypoxia decreased the turgor pressure of yellow lupin cells by 0.12 MPa (P < 0.001; Table 2), but did not affect turgor pressure of cells in wheat roots (P > 0.05; Table 2). Cells of narrow-leafed lupin roots bathed with hypoxic solution were less stable than cells in aerated roots (Fig. 6). Cells often lost turgor pressure almost immediately after puncturing and the consistency of the cell sap appeared to be altered by hypoxia, causing frequent blockages of the microcapillary.
|
Lpc of cortical cells in aerated roots was not significantly different between the species (P = 0.333), but was significantly reduced by hypoxia (P < 0.0001; Table 2). Lpc of hypoxic treated cells was lower than aerated cells, by an average of 46% for wheat, 63% for yellow lupin and 74% for narrow-leafed lupin (Table 2). Bramley et al. (2009) demonstrated that Lpc of cells in aerated wheat and lupin roots tends to increase with depth from the root surface, but the effect of hypoxia on Lpc was independent of cell location within the cortex.
Lpc influences the rate of water flow across the membrane and hence influences T1/2. T1/2 for cells in roots bathed in aerated solution ranged between 0.3 and 1.3 s, and was not significantly different between the species (P = 0.678; Table 2). For all species, mean T1/2 of cells from hypoxic roots was significantly greater than cells from aerated roots (P < 0.0001; Table 2; Fig. 6). Increase in T1/2 due to hypoxia was greater for narrow-leafed lupin root cells than for the other species (Table 2), but only five narrow-leafed lupin cells were stable for sufficient time to obtain measurements. Mean T1/2 of wheat and yellow lupin root cells doubled in response to hypoxia (Table 2), however, the effect on individual cells was variable as T1/2 ranged between 0.6–3.0 s and 0.7–4.6 s, respectively.
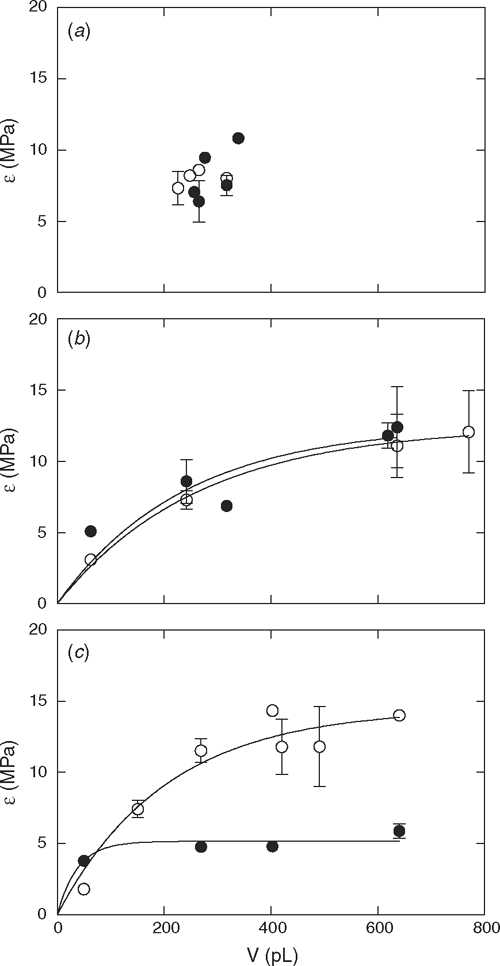
The elastic modulus of cortical cells from aerated roots ranged from 3.8 to 9.0 MPa for wheat and 1.8 to 16.7 MPa for lupins, and the average was not significantly different between the species (P = 0.705; Table 2). The variability in ॉ was due to the dependence of ॉ on cell volume (Fig. 7). Hypoxia had no effect on ॉ of wheat or yellow lupin (P > 0.05; Table 2, Fig. 7). However, hypoxia apparently reduced ॉ of narrow-leafed lupin cells, so that there was no longer a relationship between cell volume and ॉ (P = 0.0005, Fig. 7). The rate of water exchange across the cell is dependent on ॉ and therefore, a lower ॉ would result in an increase in T1/2. However, the apparent reduction in ॉ may be an artefact of the measurement process since cells with a lower ॉ may have been the only ones that were measurable due to greater membrane instability during hypoxia. When the pressure probe microcapillary is pushed against the cell wall just before puncturing the cell, it could cause a surge in turgor pressure, disrupting cells with unstable membranes. Cells with a lower ॉ would buffer against sudden changes in turgor pressure.
|
To test aquaporin activity, incubating roots with mercury and low O2 caused membranes to become leaky when punctured with the cell pressure probe; therefore, Lpc with HgCl2 during hypoxia was not measured.
Discussion
Short-term hypoxia rapidly affected transport properties in the roots of wheat and both lupin species. Root pressure changed within minutes of bathing roots with hypoxic solution, particularly lupin roots. This is a rapid response in Pr and demonstrates how sensitive roots are to their changing environment and especially how sensitive solute transport is to oxygen deficiency. The reported effects of O2 deficiency or waterlogging on water transport in roots are diverse, ranging from zero effect to more than 70% inhibition (reviewed in Bramley et al. 2007a). The source of this variability relates not only to the different species, but also to the nature of the treatment imposed. There have been few direct comparisons between species treated with the same external low O2. In this study, hypoxia affected different components of water transport in the three species, which is related to their contrasting hydraulic properties (Bramley et al. 2009) and may relate to their tolerance to waterlogging. In addition, measurements during re-aeration identified an important regulation of water transport in wheat roots, which may be beneficial for recovery in growth and the transport of water and nutrients to the shoot when flooded soils have drained. The results of this short-term study are consistent with earlier speculations (Dracup et al. 1992) based on anecdotal field observations that wheat roots are more tolerant of waterlogging than the roots of lupins. Lupin roots are more sensitive to low O2 than wheat roots, especially narrow-leafed lupin roots that become leaky.
Solute transport
The accumulation of solutes in the xylem creates an osmotic gradient that draws water into the xylem generating Pr (Steudle 1993). Solutes are able to accumulate in the apoplast of the stele because Casparian bands, located in the endodermis of all three species (Bramley et al. 2009), prevent backflow out of the stele (Steudle et al. 1993). When aerated, Pr of wheat was similar to a range of other crop species (Steudle and Jeschke 1983; Steudle and Brinckmann 1989; Azaizeh and Steudle 1991; Miyamoto et al. 2001; Lee et al. 2004). In comparison, lupins only generated low Pr when connected to the root pressure probe, similar to woody species and Lotus japonicus (Regel) K. Larsen (Hallgren et al. 1994; Steudle and Meshcheryakov 1996; Henzler et al. 1999). The difference in Pr between wheat and lupins may be related to greater active transport of ions or organic solutes in wheat roots or a lower effective reflection coefficient in lupin roots. Whilst increases in concentration of certain solutes in the xylem during flooding have been found earlier (Jackson et al. 1996), changes in Pr in response to oxygen deficiency have only previously been reported for Zea mays (L.) (Birner and Steudle 1993; Gibbs et al. 1998a). However, the response depended on the oxygen concentration because Pr of hypoxic Z. mays roots gradually recovered when re-aerated (Birner and Steudle 1993), but not roots treated for 5 h with anoxia (Gibbs et al. 1998a).
The effect of hypoxia on Pr was related to root diameter with the greatest response in narrow-leafed lupin. Root diameter is an important factor in comparisons of waterlogging tolerance as greater diffusive distances from the external medium to the root centre could lead to greater internal oxygen deficiency. Therefore, the oxygen deficiency in the stele of narrow-leafed lupin could be twice as severe as wheat, assuming similar rates of respiration. Using microelectrodes, radial profiles of the O2 partial pressure across roots have shown that the stele may be close to anoxic even with appreciable amounts of O2 in the cortex (Armstrong and Beckett 1987; Armstrong et al. 1994).
The response in Pr is likely to be due to leakage of ions and/or carbohydrates rather than an effect on energy-dependent processes because Pr began to change immediately upon O2 decreasing in the bathing medium of yellow lupin and within a few minutes of the lowered O2 for narrow-leafed lupin. Whilst the effects of hypoxia on energy-dependent solute transport to hypoxia are not slow (Trought and Drew 1980; Buwalda et al. 1988; Thomson et al. 1989), the response has not been shown to occur so rapidly. In comparison, patch clamping techniques have shown that reversible leakage of ions due to membrane depolarisation/hyperpolarisation in response to changes in aeration can occur within seconds, as has been observed for cortical cells of wheat roots (Zhang and Tyerman 1997). The process in yellow lupin roots appears to be reversible as Pr returned to original levels during re-aeration. However, the subsequent loss of Pr in narrow-leafed lupin indicates that either the endodermis became leaky or solutes leaked from the root tip. Root tips are usually the most sensitive region of roots to O2 deficiency (Drew 1997) and when waterlogged, narrow-leafed lupin roots die first at the tip (Bramley 2006).
Changes in turgor pressure provide evidence that solute leakage occurred from cortical cells of lupin roots, but not wheat, in response to hypoxia. Turgor pressure decreased indicating a loss of osmotica. Leakage from epidermal cells may also occur, but cells were physically too small to measure with the pressure probe. Unlike the effect on Pr, which was related to root diameter, cortical cells measured with the cell pressure probe were within the same cell layers and depth from the root surface for all species so the distance for O2 diffusion would have been the same. However, the instability of narrow-leafed lupin cortical cells suggests that this species is particularly sensitive to hypoxia.
Cell and root hydraulics
The influence of O2 deficiency on Lpc has only been previously measured in wheat and Z. mays (Zhang and Tyerman 1991; Tyerman et al. 1992; Zhang and Tyerman 1999). In those studies, the impact on wheat (60–85%) may have been slightly greater than the present study (46%) because cells were closer to the root tip (10–20 mm) and exposed to a slightly lower O2 concentration (0.04 mol O2 m–3). Hypoxia dramatically reduced Lpc in all species, with the effect being greater in lupins than wheat. It is unlikely that the greater reduction of Lpc in lupins was due to greater internal oxygen deficiency due to thicker roots because Lpc was measured within the same range of depth from the root surface in all species. In addition, recent evidence by Armstrong et al. (2009) indicates that respiration rates may not decline in the cortex with increasing depth into the root even when oxygen concentrations decline. Instead, the hypoxia results are analogous to those of mercury inhibition of aerated roots (Bramley et al. 2009), indicating greater inhibition of aquaporin activity in lupins than wheat. Closure of aquaporins in membranes decreases the hydraulic conductivity of membranes and hence increases the half-time of the rate of water exchange (T1/2) across the cell. Cytoplasmic pH, Ca2+ and respiration, processes that are affected by hypoxia, can regulate cell water permeability through aquaporin gating and expression (Gerbeau et al. 2002; Tournaire-Roux et al. 2003; Alleva et al. 2006; Verdoucq et al. 2008). However, although 0.05 mol O2 m–3 can induce changes in aquaporin expression within 0.5 h of treatment (Klok et al. 2002), those aquaporins that are highly expressed in roots and facilitate water transport across membranes (e.g. PIP2s, tables 2 and 5 of Bramley et al. 2007a) tend to be downregulated after a few hours of hypoxia. Given the short-term exposure of wheat and lupin roots to hypoxia, it seems more probable that Lpc was inhibited through a closure of aquaporins, rather than a reduction in their expression. What mechanism closed aquaporins is not known, but 0.5 h of zero O2 reduced cytoplasmic pH of Arabidopsis thaliana (L.) roots to 7.1 and Z. mays root tips to 6.9 (Xia and Roberts 1996; Tournaire-Roux et al. 2003). These values of pH are within the range of pH that can decrease the osmotic water permeability of plasma membranes by half due to aquaporin closure (Alleva et al. 2006; Verdoucq et al. 2008).
Despite the large reductions in Lpc in lupin roots due to hypoxia, the insignificant reduction in Lpr indicates that water flow across the cortex occurs predominantly through the apoplastic space. If flow occurred by the cell-to-cell pathway in the cortex, then Lpr would be proportionally reduced. This is consistent with Bramley et al. (2009) where Lpr of narrow-leafed lupin and yellow lupin was not affected by mercury treatment despite dramatic reductions in Lpc. This is also consistent with a smaller inhibition in Lpr compared with Lpc for Z. mays (Tyerman et al. 1992), another species believed to have predominantly apoplastic flow (Steudle and Frensch 1989), although Gibbs et al. (1998b) argue that the symplast is important. Measurements by Armstrong et al. (2009) indicate that the critical oxygen pressure (COP) at the root surface is a function of respiratory decline in the stele and that roots with larger stelar diameters should tend to have higher COPs, confirming original predictions by Berry and Norris (1949). Narrow-leafed lupin may therefore experience greater respiratory decline in the stele in comparison to the other species, when exposed to the same external oxygen concentration. However, this does not change our conclusion that bulk water flow across lupin roots bypasses cells without crossing membranes.
Using a model of a root comprising of concentric cylinders in conjunction with measurements of Lpc of each cortical cell layer and Lr, Bramley et al. (2009) identified which regions of the root contribute to Lr. In aerated wheat roots, Lr is constant with root length because water uptake preferentially occurs within 40 mm of the root tip and is probably controlled by the endodermis (Bramley et al. 2009). Changes in Lr due to aeration treatment can be predicted by altering Lpc, the length of the absorbing region and the contribution of cortical cell layers. Applying the model here shows that increasing the length of the endodermis that transports water via aquaporins reduces the inhibition by hypoxia on root hydraulic conductance (Fig. 8). Despite hypoxia inhibiting Lpc, increasing the length of the absorbing region creates more parallel pathways for flow, allowing longer roots to maintain their hydraulic conductance.
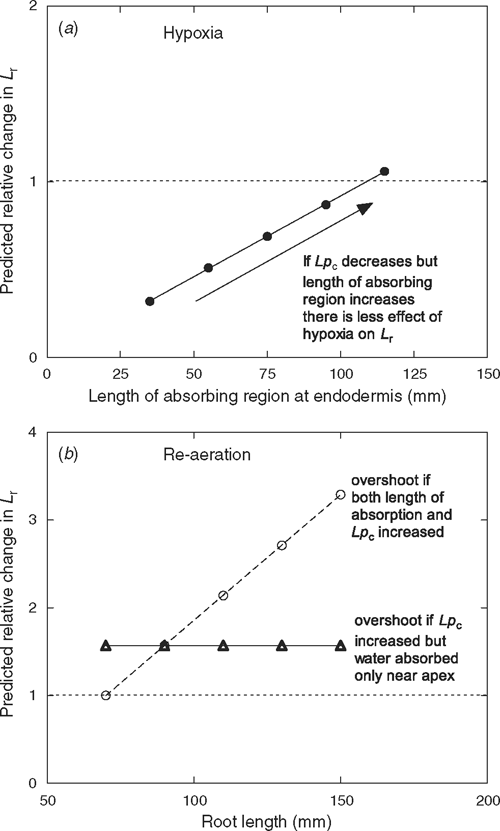
|
During re-aeration, water transport recovered in wheat roots, but with an overshoot. Overshoots in Lpr have also been observed during recovery from water deficit in Agave deserti Engelm., nutrient deprivation in wheat, hypoxia in Z. mays, and low temperature in Cucurbita ficifolia Bouché (Carvajal et al. 1996; Gibbs et al. 1998b; North et al. 2004; Lee et al. 2005), but the reason for this phenomenon has not been investigated. In this study, a greater reduction in Lpr by mercury after re-aeration, than before hypoxia, indicated that the overshoot in Lpr of wheat roots was due to greater aquaporin activity. Therefore, more aquaporins were gated open or more active aquaporins were embedded in plasma-membranes during re-aeration. Furthermore, Lpr was greater in wheat roots during re-aeration, irrespective of root length. If longer roots were absorbing water over a longer length than shorter roots, then Lr would increase with length (Fig. 8). Either Lpc returned to its original values but the length of the absorbing region was slightly longer (55 mm predicted by the model) than before hypoxic treatment, or additional pathways were opened (Fig. 8). If Lpc increased 1.6-fold during re-aeration, but only in the endodermis (35 mm, not including 5 mm apex), then Lr would be 60% greater than the value before hypoxia (Fig. 8). It would be difficult to measure such an increase in Lpc without automating the cell pressure probe, as half-times can be <1 s under ambient conditions. However, with care and a fine resolution micro-manipulator (Bramley et al. 2009), it may be possible to detect changes in Lpc in different regions of the endodermis.
The physiological significance for the effect of re-aeration could be related to the recovery of root growth when waterlogging has subsided. The rate of extension of previously waterlogged wheat roots during the recovery period was greater than control roots (Bramley 2006). An increased rate of water uptake into the cells in the zones of elongation, mediated by aquaporins, may increase the rate of root extension. In addition, an increase in the rate of water uptake after waterlogging would be needed to restore the water balance and transport of nutrients to the shoot. In comparison to wheat, lupin roots have inferior survival during waterlogging and recovery is dependent on the growth of new roots originating near the base of the stem (Bramley 2006). The hydraulics of lupin roots depends on changes in anatomy and morphology, but appears to have little ability to regulate water flow in the short term, under ambient or hypoxic conditions.
Our study focussed on the rapid responses to hypoxia using excised roots to examine their hydraulic properties. Water transport through roots is only part of the multifaceted soil–plant–atmosphere continuum that has many regulatory controls. Further research is required to link root hydraulic properties to those of shoots and functioning of whole plants. For example, although root systems of wheat and lupins are generally more sensitive to waterlogging than shoots (Trought and Drew 1980; Davies et al. 2000b), sensitivity is not solely dependent on the root system. In longer-term studies, waterlogging decreased stomatal conductance and transpiration in narrow-leafed lupin and yellow lupin, but despite closure of stomata shoot water potential of narrow-leafed lupin also declined (Davies et al. 2000c). Waterlogging also reduced leaf gas exchange in wheat plants, with a greater inhibition in a more sensitive genotype that also experienced decreased shoot water potential (Huang et al. 1994). Reductions in leaf gas exchange are believed to be caused by insufficient supply of water from the roots (Cannell and Jackson 1981), although correlations between the two processes are not clear.
Acknowledgements
We are grateful to the Grains Research Development Corporation (GRDC) of Australia and Australian Research Council for funding.
Alleva K,
Niemietz CM,
Sutka M,
Maurel C,
Parisi M,
Tyerman SD, Amodeo G
(2006) Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. Journal of Experimental Botany 57, 609–621.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Armstrong W, Beckett PM
(1987) Internal aeration and the development of stelar anoxia in submerged roots. A multishelled mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytologist 105, 221–245.
| Crossref | GoogleScholarGoogle Scholar |

Armstrong W,
Strange ME,
Cringle S, Beckett PM
(1994) Microelectrode and modelling study of oxygen distribution in roots. Annals of Botany 74, 287–299.
| Crossref | GoogleScholarGoogle Scholar |

Armstrong W,
Webb T,
Darwent M, Beckett PM
(2009) Measuring and interpreting respiratory critical oxygen pressures in roots. Annals of Botany 103, 281–293.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Azaizeh H, Steudle E
(1991) Effects of salinity on water transport of excised maize (Zea mays L.) roots. Plant Physiology 97, 1136–1145.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Belford RK,
Dracup M, Tennant D
(1992) Limitations to growth and yield of cereal and lupin crops on duplex soils. Australian Journal of Experimental Agriculture 32, 929–945.
| Crossref | GoogleScholarGoogle Scholar |

Berry LJ, Norris WE
(1949) Studies on onion root respiration. I. Velocity of oxygen consumption in different segments of roots at different temperatures as a function of partial pressure of oxygen. Biochimica et Biophysica Acta 3, 593–606.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Birner TP, Steudle E
(1993) Effects of anaerobic conditions on water and solute relations, and on active transport in roots of maize (Zea mays L.). Planta 190, 474–483.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Blackwell PS
(1983) Measurements of aeration in waterlogged soils: some improvements of techniques and their application to experiments using lysimeters. Journal of Soil Science 34, 271–285.
| Crossref | GoogleScholarGoogle Scholar |

Bramley H,
Turner DW,
Tyerman SD, Turner NC
(2007a) Water flow in the roots of crop species: the influence of root structure, aquaporin activity, and waterlogging. Advances in Agronomy 96, 133–196.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Bramley H,
Turner NC,
Turner DW, Tyerman SD
(2007b) Comparison between gradient-dependent hydraulic conductivities of roots using the root pressure probe: the role of pressure propagations and implications for the relative roles of parallel radial pathways. Plant, Cell & Environment 30, 861–874.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bramley H,
Turner NC,
Turner DW, Tyerman SD
(2009) Roles of morphology, anatomy and aquaporins in determining contrasting hydraulic behaviour of roots. Plant Physiology 150, 348–364.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Buwalda F,
Barrett-Lennard EG,
Greenway H, Davies BA
(1988) Effects of growing wheat in hypoxic nutrient solutions and of subsequent transfer to aerated solutions. II. Concentrations and uptake of nutrients and sodium in shoots and roots. Australian Journal of Plant Physiology 15, 599–612.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Carvajal M,
Cooke DT, Clarkson DT
(1996) Responses of wheat plants to nutrient deprivation may involve the regulation of water-channel function. Planta 199, 372–381.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Davies CL,
Turner DW, Dracup M
(2000a) Yellow lupin (Lupinus luteus) tolerates waterlogging better than narrow-leafed lupin (L. angustifolius). IV. Root genotype is more important than shoot genotype. Australian Journal of Agricultural Research 51, 729–736.
| Crossref | GoogleScholarGoogle Scholar |

Davies CL,
Turner DW, Dracup M
(2000b) Yellow lupin (Lupinus luteus) tolerates waterlogging better than narrow-leafed lupin (L. angustifolius). I. Shoot and root growth in a controlled environment. Australian Journal of Agricultural Research 51, 701–709.
| Crossref | GoogleScholarGoogle Scholar |

Davies CL,
Turner DW, Dracup M
(2000c) Yellow lupin (Lupinus luteus) tolerates waterlogging better than narrow-leafed lupin (L. angustifolius). II. Leaf gas exchange, plant water status, and nitrogen accumulation. Australian Journal of Agricultural Research 51, 711–719.
| Crossref | GoogleScholarGoogle Scholar |

Dennis ES,
Dolferus R,
Ellis M,
Rahman M,
Wu Y,
Hoeren FU,
Grover A,
Ismond KP,
Good AG, Peacock WJ
(2000) Molecular strategies for improving waterlogging tolerance in plants. Journal of Experimental Botany 51, 89–97.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Dracup M,
Belford RK, Gregory PJ
(1992) Constraints to root growth of wheat and lupin crops in duplex soils. Australian Journal of Experimental Agriculture 32, 947–961.
| Crossref | GoogleScholarGoogle Scholar |

Drew MC
(1997) Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology 48, 223–250.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Drew MC,
He CJ, Morgan PW
(2000) Programmed cell death and aerenchyma formation in roots. Trends in Plant Science 5, 123–127.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Enstone DE, Peterson CA
(2005) Suberin lamella development in maize seedling roots grown in aerated and stagnant conditions. Plant, Cell & Environment 28, 444–455.
| Crossref | GoogleScholarGoogle Scholar |

Gerbeau P,
Amodeo G,
Henzler T,
Santoni V,
Ripoche P, Maurel C
(2002) The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. The Plant Journal 30, 71–81.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Gibbs J,
Turner DW,
Armstrong W,
Darwent MJ, Greenway H
(1998a) Response to oxygen deficiency in primary maize roots. I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem. Australian Journal of Plant Physiology 25, 745–758.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Gibbs J,
Turner DW,
Armstrong W,
Sivasithamparam K, Greenway H
(1998b) Response to oxygen deficiency in primary maize roots. II. Development of oxygen deficiency in the stele has limited short-term impact on radial hydraulic conductivity. Australian Journal of Plant Physiology 25, 759–763.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Hallgren SW,
Rüdinger M, Steudle E
(1994) Root hydraulic properties of spruce measured with the pressure probe. Plant and Soil 167, 91–98.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Henzler T,
Waterhouse RN,
Smyth AJ,
Carvajal M,
Cooke DT,
Schäffner AR,
Steudle E, Clarkson DT
(1999) Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus. Planta 210, 50–60.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Huang B,
Johnson JW,
Nesmith S, Bridges DC
(1994) Growth, physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. Journal of Experimental Botany 45, 193–202.
| Crossref | GoogleScholarGoogle Scholar |

Jackson MB,
Davies WJ, Else MA
(1996) Pressure–volume relationships, xylem solutes and root hydraulic conductance in flooded tomato plants. Annals of Botany 77, 17–24.
| Crossref | GoogleScholarGoogle Scholar |

Kato-Noguchi H
(2000) Abscisic acid and hypoxic induction of anoxia tolerance in roots of lettuce seedlings. Journal of Experimental Botany 51, 1939–1944.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Klok EJ,
Wilson IW,
Wilson D,
Chapman SC,
Ewing RM,
Somerville SC,
Peacock WJ,
Dolferus R, Dennis ES
(2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. The Plant Cell 14, 2481–2494.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Lee SH,
Singh AP,
Chung GC,
Ahn SJ,
Noh EK, Steudle E
(2004) Exposure of roots of cucumber (Cucumis sativus) to low temperature severely reduces root pressure, hydraulic conductivity and active transport of nutrients. Physiologia Plantarum 120, 413–420.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Lee SH,
Chung GC, Steudle E
(2005) Gating of aquaporins by low temperature in roots of chilling-sensitive cucumber and chilling-tolerant figleaf gourd. Journal of Experimental Botany 56, 985–995.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Miyamoto N,
Steudle E,
Hirasawa T, Lafitte R
(2001) Hydraulic conductivity of rice roots. Journal of Experimental Botany 52, 1835–1846.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

North GB,
Martre P, Nobel PS
(2004) Aquaporins account for variations in hydraulic conductance for metabolically active root regions of Agave deserti in wet, dry, and rewetted soil. Plant, Cell & Environment 27, 219–228.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Saglio PH,
Drew MC, Pradet A
(1988) Metabolic acclimation to anoxia induced by low (2–4 kPa partial pressure) oxygen pretreatment (hypoxia) in root tips of Zea mays. Plant Physiology 86, 61–66.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Steudle E
(2001) The cohesion–tension mechanism and the acquisition of water by plant roots. Annual Review of Plant Physiology and Plant Molecular Biology 52, 847–875.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Steudle E, Brinckmann E
(1989) The osmometer model of the root: water and solute relations of roots of Phaseolus coccineus. Botanica Acta 102, 85–95.

Steudle E, Frensch J
(1989) Osmotic responses of maize roots. Water and solute relations. Planta 177, 281–295.
| Crossref | GoogleScholarGoogle Scholar |

Steudle E, Jeschke WD
(1983) Water transport in barley roots. Planta 158, 237–248.
| Crossref | GoogleScholarGoogle Scholar |

Steudle E, Meshcheryakov AB
(1996) Hydraulic and osmotic properties of oak roots. Journal of Experimental Botany 47, 387–401.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Steudle E,
Smith C, Lüttge U
(1980) Water-relation parameters of individual mesophyll cells of the crassulacean acid metabolism plant Kalanchoë daigremontiana. Plant Physiology 66, 1155–1163.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Steudle E,
Murrmann M, Peterson CA
(1993) Transport of water and solutes across maize roots modified by puncturing the endodermis. Plant Physiology 103, 335–349.
|
CAS |
PubMed |

Thomson CJ,
Atwell BJ, Greenway H
(1989) Response of wheat seedlings to low O2 concentrations in nutrient solution. II. K+/Na+ selectivity of root tissues of different age. Journal of Experimental Botany 40, 993–999.
| Crossref | GoogleScholarGoogle Scholar |

Tournaire-Roux C,
Sutka M,
Javot H,
Gout E,
Gerbeau P,
Luu D-T,
Bligny R, Maurel C
(2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425, 393–397.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Trought MCT, Drew MC
(1980) The development of waterlogging damage in young wheat plants in anaerobic solution cultures. Journal of Experimental Botany 31, 1573–1585.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Verdoucq L,
Grondin A, Maurel C
(2008) Structure–function analysis of plant aquaporin AtPIP2;1 gating by divalent cations and protons. The Biochemical Journal 415, 409–416.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Waters I,
Kuiper PJC,
Watkin ELJ, Greenway H
(1991) Effects of anoxia on wheat seedlings. II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation and sugar levels. Journal of Experimental Botany 42, 1437–1447.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Xia J-H, Roberts JKM
(1996) Regulation of H+ extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment. Plant Physiology 111, 227–233.
|
CAS |
PubMed |

Zhang W-H, Tyerman SD
(1991) Effect of low O2 concentration and azide on hydraulic conductivity and osmotic volume of the cortical cells of wheat roots. Australian Journal of Plant Physiology 18, 603–613.
| Crossref | GoogleScholarGoogle Scholar |

Zhang W-H, Tyerman SD
(1997) Effect of low oxygen concentration on the electrical properties of cortical cells of wheat roots. Journal of Plant Physiology 150, 567–572.
|
CAS |
PubMed |

Zhang W-H, Tyerman SD
(1999) Inhibition of water channels by HgCl2 in intact wheat root cells. Plant Physiology 120, 849–858.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |