Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update
Claudio Lovisolo A C , Irene Perrone A , Andrea Carra A , Alessandra Ferrandino A , Jaume Flexas B , Hipolito Medrano B and Andrea Schubert AA DCA, Plant Physiology, University of Turin, via Leonardo da Vinci 44, 10095 Grugliasco, Italy.
B Grup de Recerca en Biologia de les Plantes en Condicions Mediterranies, Universitat de les Illes Balears, Carretera de Valldemossa Km 7.5, 07122 Palma de Mallorca, Spain.
C Corresponding author. Email: claudio.lovisolo@unito.it
Functional Plant Biology 37(2) 98-116 https://doi.org/10.1071/FP09191
Submitted: 23 July 2009 Accepted: 6 November 2009 Published: 3 February 2010
Abstract
This review deals with grapevine responses to water stress by examining perturbations to physiological and molecular processes at the root, shoot, leaf and berry levels. Long-distance signalling among organs is also considered. Isohydric or anisohydric Vitis genotypes are described in relation to their response to drought, which is linked to stomatal behaviour. Stomatal regulation of grapevine under abscisic acid and hydraulic control (the latter being linked to embolism formation and recovery in water pathways upstream the stomata) is reviewed and linked to impairments of photosynthetic assimilation. We define three stages of photosynthesis regulation in grapevines that are subjected to progressive water stress on the basis of the main causes of assimilation decline. Early and late contributions of aquaporins, which play a fundamental role in water stress control, are discussed. Metabolic mechanisms of dehydration tolerance are rewieved, and variation linked to differences in transcript abundance of genes involved in osmoregulation, photosynthesis, photorespiration, detoxification of free radicals and coping with photoinhibition. Results of these defence strategies accumulated in berries are reviewed, together with perturbations of their molecular pathways. Features observed in different organs show that grapevine fits well as a complex model plant for molecular and physiological studies on plant drought avoidance/tolerance.
Additional keywords: abscisic acid, anisohydric, anthocyanins, aquaporin, genome, isohydric, polyphenols, proteomics, stomatal conductance, transcriptomics, water use efficiency.
Introduction
Advances in the understanding of plant responses to water deficits require information from the molecular level to that of whole plant (Chaves et al. 2003). Grapevine has been used as model plant to study ecophysiological responses to water stress since the 1970s. Molecular studies began in the 1990s, and, more recently, applications of ‘-omics’ technologies, such as transcriptomics and proteomics, together with the availability of the grapevine genome sequence (Jaillón et al. 2007; Zharkikh et al. 2008) have provided a powerful molecular counterpart to physiological analyses (Troggio et al. 2008). This review uses a physiological platform to incorporate recent molecular developments to provide an understanding of how grapevine responds to and recovers from drought stress.
Grapevine is grown widely throughout the world, with its production making the top agriculture lists in many countries (Bisson et al. 2002). According to FAOSTAT time-series and cross-sectional data (http://faostat.fao.org, accessed September 2009) there were 66 271 676 tonnes of grapes produced on 7 501 872 ha in 2007.
Vitis vinifera L. cultivation is traditionally non-irrigated (especially in Europe) and spread widely across dry and semi-dry ecosystems. Yield and berry quality depend strongly on the vine adaptability to drought. Water stress does not imply exclusively negative effects, but a regulated water stress, which is the base of various agronomic practices (e.g. rootstock use, controlled cover crops, tillage, rescue irrigation techniques such as regulated deficit irrigation or partial root-zone drying) has been largely used to balance vine vegetative and reproductive growth with the aim of controlling berry quality. Understanding and manipulating plant–water relations and water-stress tolerance by means of physiology and molecular biology can significantly improve plant productivity and environmental quality, hence, is clearly of wide economic importance in viticulture.
In this review we examine root, shoot, leaf and berry, linking physiological and molecular knowledge to applied research. We focus on the role of aquaporins in water movement and how water constraints modify organ functioning and generate signals directed to other plant organs. Drought signalling among grapevine organs has a dual component: a hydraulic signal controlled by xylem physiology coexists with chemical signals (involving hormones), transported via xylem, phloem and parenchyma pathways (Loveys 1984b; Lovisolo et al. 2002a). Grapevine is a model species to study the different drought signalling mechanisms, as many cultivars and genotypes are available, including the different species of the genus Vitis.
Root and shoot sensing of water stress
When roots sense a soil water deficit, root cells respond in terms of their growth and differentiation, and also transmit a signal that is then perceived in the shoot. The response to drought involves first the acidification of the apoplast by protons pumped from the cytoplasm. This acidification stimulates the action of expansins, important in cell wall synthesis. Of the 10 expansin genes in grapevine, Vlexp2 isolated from Vitis labruscana (Vitis labrusca L. × V. vinifera L.) cv. Kyoho berries, showed high root expression level (Ishimaru et al. 2007). From V. vinifera cv. Arka Neelamani, Thomas et al. (2003) isolated VvPRP1 and VvPRP2, two genes encoding a distinct type of proline-rich proteins (PRPs), a major category of cell wall proteins in plants, involved in different developmental and environmental responses to abiotic stresses. Grape PRP genes are expressed in root tissues and not detected in shoot tip or leaf tissues; they play a role in the initiation of new roots on grape stem cuttings, probably by altering the cell wall mechanical properties to enable root emergence.
Although a reduction in leaf and shoot growth is one of the signs of grapevine water deficit (Stevens et al. 1995), the decrease in root growth is less than shoot growth (Dry et al. 2000a, 2000b). This results in a higher root : shoot ratio, which ensures adequate water and nutrient transport to the shoots. The tolerance of grapevines to drought has often been attributed to their ability to produce new roots selectively where soil water is available (Morlat and Jacquet 1993; Dry et al. 2000b). Droughted roots continue to grow into deeper, wetter soil layers, whereas the roots of irrigated plants proliferate mostly in the topsoil (Bauerle et al. 2008). As deeper roots procure soil-mobile nutrients, like nitrate (NO3–) (Keller 2005), this mechanism also enhances plant nitrogen uptake.
Shoot growth inhibition comprises inhibition of internode extension, leaf expansion and elongation of tendrils (Schultz and Matthews 1988; Hardie and Martin 2000) and has been used as a sensitive indicator of grapevine water status (Pellegrino et al. 2005; Lebon et al. 2006). In addition, comparably low water stress levels induce a decrease in the average diameter of grapevine vessels and a decrease of xylem hydraulic conductivity (Lovisolo and Schubert 1998; Lovisolo et al. 2002b). Although shoot growth inhibition limits transpiration, reduced vessel size prevents excessive loss of water by reducing xylem conductivity and may help to prevent embolisation, as smaller vessels are less susceptible to cavitation (Salleo et al. 1985). Extreme drought consequences at the shoot level involve shedding of leaves and cessation of secondary growth, as reviewed by Keller (2005).
V. vinifera Grenache and Chardonnay plants show a higher amount of suberin deposited in cell walls of droughted roots than in well watered roots in response to water stress, both at endodermal and exodermal levels. In Grenache, all cells of the endodermis appear to become suberised, whereas in Chardonnay some passage cells are still evident. These cellular adaptations assist in modulating stress responses in these two grape cultivars (Vandeleur et al. 2009).
In addition to root growth and apoplastic barrier formation, plants can modulate responses to soil water stress by comparmentalising the water transport pathways which are under metabolic control. This implies that roots can either modulate water-driving cell osmotic forces and/or regulate expression and activity of aquaporins (Galmés et al. 2007; Lovisolo et al. 2008b).
Aquaporins are thought to control the radial movement of water through roots (Maurel et al. 2008) and through living tissues adiacent to root and shoot xylem cells (Kaldenhoff et al. 2008). Some aquaporins are constitutively expressed (Johansson et al. 2000) but the expression of others is regulated by different stimuli, such as adverse environmental conditions (Vera-Estrella et al. 2004). Indeed, expression of different aquaporin genes may be stimulated, decreased or unchanged under abiotic stress (Kaldenhoff et al. 2008). Grapevine aquaporins have been cloned by Baiges et al. (2001), Picaud et al. (2003), Perrone et al. (2006), Reid et al. (2006), Galmés et al. (2007), Fouquet et al. (2008), Glissant et al. (2008), Schlosser et al. (2008), Shelden et al. (2009) and Vandeleur et al. (2009), and reported to be expressed either in roots or shoots.
The role of aquaporins in root water transport was investigated by comparing grapevine cultivars that differed in water use strategies, as described by Vandeleur et al. (2009). These workers studied gene expression of VvPIP1;1 and VvPIP2;2, the two PIP aquaporins with higher expression in roots of both Chardonnay and Grenache plants. VvPIP2;2 showed a constitutive expression in root regardless of cultivar or soil water condition, whereas VvPIP1;1 transcript expression increased during water stress in Chardonnay, but not in Grenache. Functional assays showed a gain of water permeability (Pos) when VvPIP2;2 and VvPIP1;1 cRNA were injected together in the oocytes but not when VvPIP2;2 was injected alone. Hence, it was proposed that Chardonnay plants show a minor reduction of root hydraulic conductance caused by water stress through increased transcellular component of radial water transport. This provides water while transpiration continues; thus, an anisohydric behaviour (see below) is reported for this cultivar. In this way, Chardonnay plants are able to maintain lower water potential differences between soil and xylem, possibly due to lower vulnerability to embolism formation compared with Grenache, as described by Alsina et al. (2007).
Grenache plants demonstrate a different strategy: the finding of no variation in transcript level of most important root PIP aquaporins and suberisation implies a lower hydraulic conductance in water deficit conditions. This supports the hypothesis of tight control on stomatal regulation that is typical of isohydric (see below for explaination) cultivars like Grenache, which aims to avoid excessively negative xylematic water potential and, therefore, cavitation (Schultz 2003a; Soar et al. 2006; Vandeleur et al. 2009). It is hypothesised that in the isohydric Grenache, the drought-induced root abscisic acid (ABA) biosynthesis increases apoplastic concentration because of a concomitance of events: an increase of suberisation of apoplastic barriers causes a reduction of water conductivity which is not compensated by aquaporin-mediated water transport.
Literature reviewed in this section shows a lack of experiments dealing with root hydraulics; grapevine root aquaporins are, in some cases, performed on Vitis spp. rootstocks grafting V. vinifera scions (e.g. Schultz 2003a; Alsina et al. 2007), whereas others are performed on own-rooted varieties of V. vinifera (e.g. Soar et al. 2006; Vandeleur et al. 2009). However, the iso- or anisohydric response is always detected at the scion level, showing that a strict causal linkage of the scion response with root aquaporins occurs only on own-rooted plants.
Embolism formation and removal: a phenomenon affecting hydraulics and transpiration of grapevines
Embolism of xylem vessels as a result of water stress has been extensively studied in grapevines. Under high levels of tension, gas-filled xylem vessels may become disrupted by breakage of water columns, which causes embolism formation and drastically reduces hydraulic conductance (Schultz and Matthews 1988; Lovisolo and Schubert 1998). In different studies comparing root and shoot embolisms, roots have been shown to be more vulnerable to xylem cavitation than shoots (Lovisolo and Schubert 2006; Lovisolo et al. 2008a). This protects the stem from extreme xylem tensions during severe drought (Sperry et al. 2006). Froux et al. (2005) demonstrated that lateral roots seem to be more prone to embolism than the main root, suggesting a behaviour similar to ‘hydraulic segmentation’ in petioles. In plants with an anisohydric type response to drought, like Populus euphratica (Hukin et al. 2005), a lower cavitation vulnerability of the roots compared with shoots was found. In P. euphratica stomatal closure occured relatively late, well after shoot hydraulic conductance was significantly affected by embolisms, showing that in the anisohydric response to drought a lower vulnerability of roots to cavitation may be part of the survival strategy.
Although grapevine petioles and roots are more vulnerable to embolism than shoots (Lovisolo et al. 2008a), when plants are rewatered, either root or shoot and petioles recover ~35–40% of hydraulic conductivity within 24 h, suggesting that a common and coordinated mechanism of recovery among all plant organs occurs. The proposed mechanism is based on three points: (i) embolism repair occurs progressively in shoot and then in root and in petioles, following an almost full recovery of leaf water potential; (ii) hydraulic conductance recovery in all plant organs also occurs during diurnal transpiring hours when formation and repair of embolisms occurs in all plant organs; and (iii) a non-hydraulic stress-derived ABA residual signal in rehydrated leaves hinders stomatal opening even when leaf water potential and the overall plant hydraulics are recovered, suggesting that an ABA-induced transpiration control promotes gradual embolism repair in rehydrated grapevines (Lovisolo et al. 2008a).
To reintegrate vessel functionality, plants have developed different repair mechanisms, which, in some cases, are associated with positive root pressure, and in other cases involve active and energy-consuming processes in shoot conductive tissues (Salleo et al. 2004). In grapevine, experiments conducted with the metabolic and water transport inhibitor mercuric chloride point to the presence of an active mechanism involving the contribution of living cells (Lovisolo and Schubert 2006), and also possibly involving the contribution of aquaporins. A role of aquaporins in embolism repair was previously hypothesised based on asymmetrical aquaporin distribution, which appeared enriched at the interface between xylem vessels and associated living cells (Kaldenhoff et al. 2008). Aquaporin distribution in grapevine cells has not yet been described, although in water-stressed grapevine shoots the expression of an aquaporin (PIP1) was found to be downregulated (Cramer et al. 2007). However, aquaporins can be activated by post-translational modifications, e.g. by phosphorylation, thus, their role in embolism refilling cannot be ruled out on the basis of expression studies.
Aquaporins may be involved in embolism repair. In walnut (Juglans regia L.) PIP2 aquaporins, localised in xylem vessel parenchyma cells, were activated during spring embolism recovery (Sakr et al. 2003). PIP1 and PIP2 antisense Arabidopsis plants were slower than controls in the conductance recovery after rewatering (Martre et al. 2002). Tobacco (Nicotiana tabacum L.) RNAi plants showing an impaired expression of PIP1 or PIP2 genes led to a direct evidence of aquaporin involvement in embolism recovery. PIP1-RNAi and control plants repaired embolism in a few hours after rehydration, but PIP2-RNAi plants showed a delayed kinetics of recovery (Kaldenhoff et al. 2008).
Results of recent work suggest ABA/aquaporin interaction in embolism repair and in aquaporin activation during water stress (Kaldenhoff et al. 2008). ABA may be involved in gating mechanisms of water channels by facilitating their structural restoration, possibly acting from the cytoplasmatic side of aquaporins (Wan et al. 2004). Although downregulation of aquaporins after ABA treatment has been reported (Mariaux et al. 1998; Suga et al. 2002), more often aquaporins, if responsive, were upregulated by this hormone (Jang et al. 2004). Parent et al. (2009) obtained sense and antisense transgenic maize (Zea mays L.) plant for 9-cis-epoxycarotenoid dioxygenase NCED/VP14 gene that catalyses the first specific step in ABA biosynthesis. Expression levels of most root PIP isoforms were significantly higher in sense plants than in antisense plants, and analogous results were obtained for the influence on protein content. Moreover, a long-lasting effect was observed. This transgenic approach allows us to distinguish between ABA and non-ABA effects on aquaporins.
Stomatal and non-stomatal limitations to photosynthesis
Under conditions of high irradiance and vapor pressure deficit (e.g. midday of clear summer days), water flow into grapevine leaves, as in many other species, is insufficient to compensate water losses through evapotranspiration, resulting in a midday to afternoon depression of leaf water potential (Schultz 2003a; Chaves et al. 2007). As a consequence, midday to afternoon depression of stomatal conductance (gs) and net photosynthesis (AN) has been reported in many cultivars, even under sufficient soil water availability (Gómez-del-Campo et al. 2004; Moutinho-Pereira et al. 2004). Differences among cultivars in stomatal responsiveness to midday conditions have been described (Winkel and Rambal 1990).
Midday stomatal closure is often associated with accumulation of ABA in the petiole xylem and leaves (Loveys 1984a; Rodrigues et al. 2008), although increased xylem pH (Rodrigues et al. 2008) and decreased plant hydraulic conductance (Salleo and Lo Gullo 1989; Vandeleur et al. 2009) could also be involved. However, stomatal closure is not the only cause of decreased photosynthesis during the midday depression. The depression involves both stomatal and non-stomatal factors, as reflected by lower photosynthesis during the afternoon than in the morning at any given stomatal conductance (Cuevas et al. 2006) or substomatal CO2 concentration (Downton et al. 1987; Quereix et al. 2001). In addition, gs in the afternoon is less sensitive to ABA and more sensitive to CO2 than during the morning, also supporting the idea that reduced photosynthesis is limiting gs in the afternoon and not vice versa (Düring 1991; Correia et al. 1995).
Non-stomatal limitations may also be partly responsible for the midday depression. Photoinhibition, feedback inhibition through source–sink interactions, and decreased mesophyll conductance to CO2 have been suggested as important limiting factors, but none of these processes has been demonstrated to predominate (Flexas et al. 2008). For instance, Escalona et al. (2003) showed that, in irrigated plants, a midday depression occurs in the most exposed leaves of the canopy, but not in shaded leaves. Leaves exposed to constant high light, temperature and vapor pressure deficit had a maximum stomatal conductance 1 h after illumination, which declined thereafter (Correia et al. 1990; Lu et al. 2003). Also, using remote sensing of chlorophyll fluorescence, Flexas et al. (2000) showed that the quantum efficiency of PSII was lower during the afternoon than during the morning at any given light intensity. All these observations suggest a possible involvement of photoinhibition. Indeed, the maximum quantum efficiency of PSII (Fv/Fm) declines slightly at midday, although typically less than 20% (Quick et al. 1992; Bertamini and Nedunchezian 2004). Bertamini and Nedunchezian (2004) reported that this decline was initially (2 h after high light exposure) associated with a decrease in the concentration of the D1 protein (the core protein of PSII components), and later (4 h after high light exposure) with a decline of the 33KDa protein (the water-splitting complex) while D1 was recovered, suggesting that both acceptor side and donor side photoinhibition are involved in midday depression of photosynthesis. However, in other cases were Fv/Fm declines somewhat, it correlates better with the accumulation of de-epoxidated xanthophylls than with degradation of D1 protein (Chaumont et al. 1995). The mechanism of repair of D1 is very effective in grapevines; in its presence, the fraction of functional PSIIs is kept higher than 50% (and Fv/Fm between 0.6 and 0.8) even exposing leaves to photon exposures higher than those of a normal sunny day. Conversely, when the D1 repair is blocked with lincomycin the fraction of functional PSIIs and Fv/Fm rapidly decline to zero with photon exposures similar to those received by a grapevine at midday (Flexas et al. 2001). Moreover, in other experiments, Fv/Fm remained constant during the day (Iacono and Sommer 1996; de Souza et al. 2003), and there was no degradation of chlorophyll (Medrano et al. 2002a), suggesting that effective photoprotection is associated with midday depression of photosynthesis. Chlorophyll fluorescence data corroborate this view, showing midday to afternoon decline in the steady-state fluorescence emission and a constant or slightly declined in the effective quantum efficiency of PSII (φPSII) and rate of electron transport (ETR) (Correia et al. 1990; Flexas et al. 2000). Meanwhile, non-photochemical quenching parameters (qP, NPQ) largely increase and the photochemical reflectance index (PRI) decreases (Evain et al. 2004). These changes are associated with increased trans-thylakoid ΔpH and de-epoxidation of xanthophylls, reflecting safe thermal energy dissipation (Düring 1999; Medrano et al. 2002a; Evain et al. 2004). The rate of photorespiration increases or is kept constant in the afternoon in irrigated plants (Iacono and Sommer 1996; Flexas et al. 1999a, 2000), while the foliar pools of ascorbate and glutathione slightly increase, and their oxidised forms increase over the reduced forms (Chaumont et al. 1995). Together these mechanisms confer an effective photoprotection to grapevines leaves during the midday depression of photosynthesis.
Alternatively, a phloem-based feedback signal could be involved in the regulation of the balance between source and sinks activities, leading to an afternoon decline of photosynthesis (Quereix et al. 2001). This would be supported by the observed accumulation of sucrose and starch in leaves during the course of the day (Chaumont et al. 1994), although in other experiments they rather remain constant or even decrease (Quick et al. 1992). Alternatively, a decrease in mesophyll conductance to CO2 (gm) concomitant to decreased gs, as observed by Moutinho-Pereira et al. (2004) in three different grapevine cultivars, may result in a further decrease in chloroplast CO2 availability, explaining the non-stomatal component of photosynthesis reductions.
Regulation of leaf water potential: isohydric and anisohydric cultivars
In the next section we provide a generalised view of grapevine leaf responses to water stress.
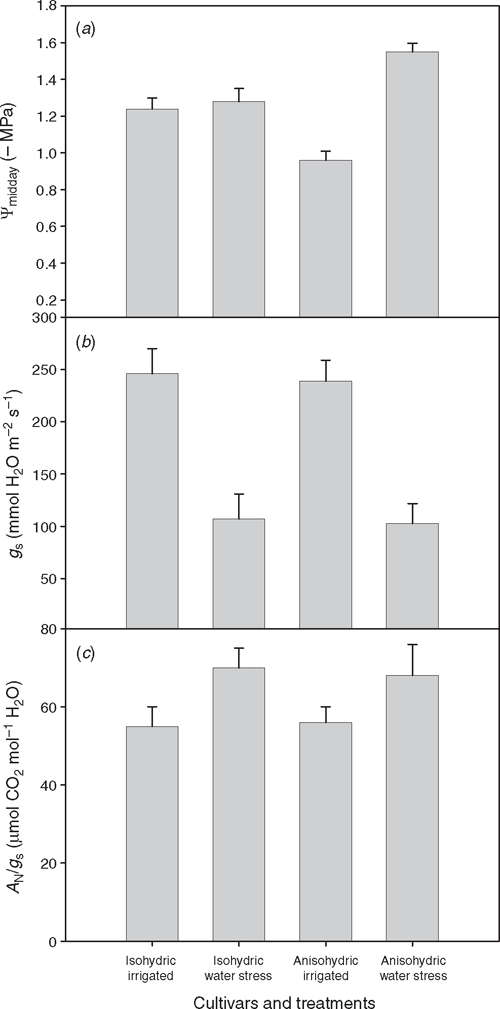
When soil water stress combines with high atmospheric water demand, reductions in leaf water potential and gas exchange become larger and longer-lasting (Liu et al. 1978; Flexas et al. 1998; Schultz 2003a; Pou et al. 2008). Based on their water potential behaviour in response to water stress, grapevine cultivars have been classified as isohydric or anisohydric (Schultz 2003a; Vandeleur et al. 2009). Isohydric cultivars are those that keep their leaf water potential above a certain threshold regardless of soil water availability or atmospheric water demand. Anisohydric cultivars are those in which leaf water potential drops with decreasing soil water availability or increasing atmospheric water demand (Fig. 1a). In isohydric grapevines, leaf water potential rarely drops below –1.5 MPa (Fig. 1a). This is close to the threshold for severe cavitation in this species (Salleo and Lo Gullo 1989; Lovisolo et al. 2008a), although some cavitation occurs at lower water potentials in petioles, shoot nodes and internodes and roots (Schultz and Matthews 1988; Salleo and Lo Gullo 1989; Schultz 2003a; Lovisolo et al. 2008a). According to observations in other isohydric species as laurels (Laurus nobilis L.) (Salleo et al. 2000), it may be argued that these cultivars present a fine co-regulation of gs and leaf hydraulic conductivity, allowing them to avoid cavitation. Grapevines typically reported as isohydric include the hybrid species V. labruscana (Vitis labrusca × V. vinifera) and the rootstock Richter-110 (Vitis berlandieri Planch. × Vitis rupestris Scheele), widely used V. vinifera cultivars such as Grenache, Trincadeira Preta and Tempranillo, as well as some cultivars native to dry viticultural areas. Anisohydric cultivars, by contrast, drop leaf water potential through the day as a function of soil water deficit (Fig. 1a), which is often achieved by means of osmotic adjustment (Downton 1983; Düring 1984; Schultz and Matthews 1993; Patakas and Noitsakis 1997), although may also be through changes in cell wall elasticity (Patakas and Noitsakis 1997). Grapevines typically reported as anisohydric include the species V. labrusca and Vitis californica Benth., as well as many V. vinifera cultivars, including Chardonnay, Cabernet Franc, Cabernet Sauvignon, Syrah, Riesling, Carignan, Muscat, Thomson seedless, Touriga Nacional, as it appears from a literature survey (references in the legend of Fig. 1).
|
Although it has been suggested that differences between iso- and anisohydric cultivars may include different stomatal responses and water use (Schultz 2003a), on average, they show similar decreases in gs (Fig. 1b) and AN (data not shown) in response to water stress. As a consequence, both iso- and anisohydric cultivars present similar values of leaf intrinsic water-use-efficiency (AN/gs) both under irrigation and under water stress (Fig. 1c). Indeed, differences among grapevine cultivars in water use efficiency (WUE) have been reported, based on instantaneous gas-exchange data (Gómez-del-Campo et al. 2004), isotopic composition (13C/12C) of leaf and/or fruit dry matter (Gibberd et al. 2001; Gaudillère et al. 2002) or biomass accumulation per unit of water used (Gibberd et al. 2001). However, no clear pattern of correlation is observed in these studies between WUE and the iso- or anisohydric character of the studied cultivars.
Moreover, the same cultivar can behave as iso- or anisohydric, depending on the conditions. For instance, V. labruscana was reported to be anisohydric by Liu et al. (1978), but as isohydric by Naor and Wample (1994). Pinot Noir behaves as anisohydric when water stress is applied pre-véraison and as isohydric when it is applied post-véraison (Poni et al. 1993). In contrast, during most of the growing season (June–July), Tempranillo and Manto Negro are often reported as isohydric (Flexas et al. 1998; Medrano et al. 2003), but later in the season (August), however, they behave as iso- or anisohydric, depending on the year (J. Flexas and H. Medrano, unpubl. data). However, the cavitation threshold of –1.5 MPa described for grapevines (Salleo and Lo Gullo 1989) is rarely and barely reached even during the ‘anisohydric years’, suggesting that grapevines are effective in avoiding catastrophic cavitation.
Soil water stress and mechanisms of stomatal closure
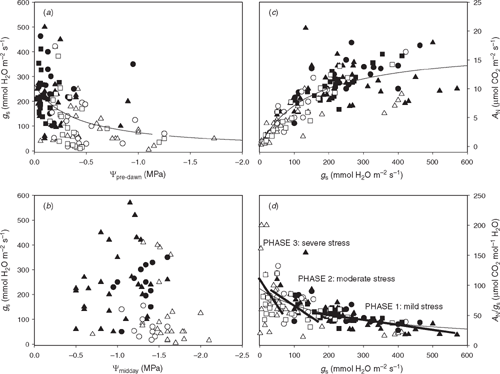
Stomatal closure is one of the first processes that occurs in the leaves in response to soil water stress. Many signals are involved in sensing environmental variations during soil drought-induced stomatal closure. They are related more to ABA metabolism, hydraulic signals (cavitation), regulation of expression and activity of aquaporins, and/or electric signals, than changes in leaf water status, measured as leaf water potential. Indeed, with some exceptions, stem water potential has been described as a more preferable indicator of grapevine water status than leaf water potential (Choné et al. 2001; Intrigliolo and Castel 2006; Williams and Baeza 2007), and gs often correlated better with leaf water potential determined at pre-dawn (Ψpre-dawn) than Ψmidday (Winkel and Rambal 1990; Schultz 2003b; Intrigliolo and Castel 2006). This is also observed in the survey of iso- and anisohydric cultivars used in Fig. 1 (Fig. 2). Although a significant correlation is found when plotting midday gs against Ψpre-dawn, pooling all the data together (Fig. 2a), no clear relationship is observed between gs and Ψmidday (Fig. 2b), even if only anisohydric cultivars (triangles) are considered. These observations suggest that gs in grapevines decreases under water stress in response to some root- or stem-based signal(s). Indeed, this is the basis of the partial root drying technique used to reduce vegetative growth and water use and improve WUE in grapevines (Dry and Loveys 1998; Chaves et al. 2007).
|
Grapevines were among the first plant species in which a direct role of ABA in stomatal closure was demonstrated (Loveys and Kriedemann 1974; Liu et al. 1978; Loveys 1984a, 1984b). In different grapevine genotypes during the gradual imposition of soil water stress (non-irrigation) or partial root drying, tight negative correlations are often observed between gs and either xylem (Pou et al. 2008; Rodrigues et al. 2008) or leaf tissue (Loveys and Kriedemann 1974; Liu et al. 1978; Lovisolo et al. 2002a) ABA contents. On the basis of these observations it is often assumed that root ABA synthesis in response to water stress and transport through the xylem into leaves mediates most of the stomatal response in grapevines, although a reverse transport, i.e. from leaves to roots has also been shown (Loveys 1984b). However, gradients in both xylem and leaf ABA along shoots of grapevines, from higher concentrations close to the apex to lower concentrations downwards have been observed (Soar et al. 2004). These gradients, which resulted in a gradient of gs among leaves with different positions along the shoot, are inconsistent with the concept of root-derived ABA. Analysing the patterns of expression along the shoots of two genes involved in ABA synthesis, Soar et al. (2004) concluded that the observed differences in ABA were due to differences in the in situ synthesis of ABA in shoots and leaves. Soar et al. (2006) further confirmed that regulation of gene expression in response to increased vapour pressure deficit in leaf tissue and not in roots was associated with the higher xylem ABA concentrations found in Grenache than in Syrah. In Arabidopsis, it has been clearly demonstrated that gs response to soil water stress is due to increased ABA synthesis in the shoots, not in the roots (Christmann et al. 2007). The observations of Soar et al. (2004, 2006), although not conducted on water stressed plants, suggest that this may also be the case in grapevines.
That ABA synthesis in the shoots and leaves increases in response to soil water stress implies that some other root-based signal may trigger this response. Other hormonal signals have been suggested, such as phaseic acid (Loveys and Kriedemann 1974) or cytokinins (Stoll et al. 2000), as well as the influence of xylem sap pH (Rodrigues et al. 2008), but no such roles have been confirmed. In contrast, in Arabidopsis the signal triggering an increase of shoot ABA synthesis has been shown to be of hydraulic nature (Christmann et al. 2007). Furthermore, there is a gradient of hydraulic conductivity in nodes and internodes along grapevine shoots, similar to the gradient described for ABA synthesis (Salleo et al. 1982, 1985; Lovisolo and Schubert 1998). In internodes, both xylem hydraulic conductivity (Kh) and leaf specific conductivity (LSC) are lower in the most apical parts, but in lower internodes they are similar (Salleo et al. 1982, 1985) or increase slightly from the shoot base to intermediate shoot positions, thereafter declining towards the apex (Lovisolo and Schubert 1998). In the nodes, LSC is often lower than in internodes and continuously declines from the base to the apex (Salleo et al. 1982). This distribution of hydraulic conductivity could explain why apical leaves synthesize more ABA and have lower gs (Soar et al. 2004). There is evidence that hydraulic conductivity of roots, shoot nodes and internodes and petioles, as well as whole-plant conductivity decline in grapevines subjected to water stress (Schultz and Matthews 1988; Salleo and Lo Gullo 1989; Winkel and Rambal 1993; Lovisolo and Schubert 1998, 2006; Lovisolo et al. 2002a, 2008a, 2008b; Schultz 2003a; Pou et al. 2008). This decline can be due to water stress-induced changes in xylem development (Mapfumo et al. 1993; Mapfumo and Aspinall 1994), but most often occurs by means of drought-induced cavitation (Schultz and Matthews 1988; Salleo and Lo Gullo 1989; Schultz 2003a). Roots and leaf petioles appear to be more sensitive than shoots to drought-induced cavitation (Schultz 2003a; Lovisolo et al. 2008a), although in the shoots, internodes are much more sensitive than nodes (Salleo and Lo Gullo 1989). In addition to cavitation, aquaporins have been suggested to play a role in the regulation of hydraulic conductivity in grapevines. All known aquaporins in grapevines are much more abundant in roots than in any other tissue, although they are still present in shoots and leaves. Experiments of gene expression during drought and recovery in Richter-110 have shown that several PIP and TIP aquaporins are upregulated at early stages of water stress in roots, while they are mostly downregulated in leaves (Galmés et al. 2007). In contrast, after re-watering stressed plants, most aquaporins are upregulated in leaves but not in roots (Galmés et al. 2007). Using mercury as an inhibitor of the activity of some aquaporins, it has been suggested that aquaporins are involved in the recovery after water stress of shoot (Lovisolo and Schubert 2006) and root (Lovisolo et al. 2008b) hydraulic conductivity. Using this technique it has been shown that in drought-resistant rootstocks, aquaporin-regulated decrease of Kh is more important than cavitation, the opposite being true for drought-sensitive rootstocks. Moreover, it has been suggested that a reduced transpiration induced by ABA after re-watering promotes aquaporin-mediated embolism repair after water stress (Lovisolo and Schubert 2006; Lovisolo et al. 2008a).
Regardless of the mechanism for its regulation, whole-plant hydraulic conductivity often correlates well with gs during drought imposition (Winkel and Rambal 1993; Schultz 2003a; Pou et al. 2008), and much better during recovery after water stress (Lovisolo et al. 2008a; Pou et al. 2008), suggesting that gs may be regulated by hydraulic signals during water stress. However, in a factorial experiment involving water stress, partial root drying and downwards shoot position, Lovisolo et al. (2002a) presented clear evidence that it was leaf ABA and not whole-plant hydraulic conductivity that determines gs in grapevines. Further work is required to understand the role of hydraulics on stomatal regulation in grapevines.
Alternatively, electrical signalling could be involved in stomatal regulation in grapevines, as suggested in other species (Fromm and Fei 1998; Grams et al. 2007). In grapevines, the two types of wound-induced electrical signals, namely variation potentials (VPs) and action potentials (APs), propagate very fast (~3 mm s–1 and 100 mm s–1 for VPs and APs, respectively) in leaf tissues (Mancuso 1999). Preliminary data (J. Fromm and J. Flexas, unpubl. data) showed that re-watering water stressed grapevine plants generates APs that are transmitted very quickly (less than 10 s) from roots to leaves, similar to the observations in Zea mays, where the electrical rather than the hydraulic signal was shown to induce stomatal re-aperture (Grams et al. 2007).
Gradual downregulation of photosynthesis under soil water stress
As in other species, a tight curvilinear correlation between gs and AN has been described in grapevines (Flexas et al. 2002a). This relationship is also observed when pooling together all data of the survey described in previous sections (Fig. 2c). The observed dispersion may depend on temperature and atmospheric vapour pressure deficit (Zufferey et al. 2000), leaf age (Zufferey et al. 2000) and time of the day or season (Gómez-del-Campo et al. 2004; Cuevas et al. 2006), but primarily due to intrinsic differences among cultivars (Düring 1987; Bota et al. 2001).
Due to the tightness of this correlation and to the fact that intrinsic WUE (i.e. photosynthesis to gs ratio) increases as gs decreases (Fig. 2d), it is often assumed that the drought-induced decrease of photosynthesis is mediated by stomatal closure. Indeed, under mild water stress this may be the case. Using the daily maximum value of gs as an indicator of water stress that allows comparison of plants with iso- and anisohydric behaviours, Flexas et al. (2002b) and Medrano et al. (2002b) defined several stages of photosynthesis regulation in grapevines subjected to progressive soil water stress on the basis of the main causes of AN decline. These stages are general regardless of the cultivar under study and are as follows:
-
Stage 1. Mild water stress: gs decreases from a maximum (typically 200–500 mmol H2O m–2 s–1 in grapevines) to 150 mmol H2O m–2 s–1.
-
Stage 2. Moderate water stress (or transition stage): gs ranges between 50 and 150 mmol H2O m–2 s–1.
-
Stage 3. Severe water stress: gs drops below 50 mmol H2O m–2 s–1.
At Stage 1, the effects of water stress consist in a relatively small decline of AN, which results in a progressive increase of intrinsic WUE (Fig. 2d) and a slight decline of substomatal CO2 concentration (Ci). As a consequence of decreased CO2 availability in the mesophyll, the rate of photorespiration increases somewhat (Flexas et al. 1999a, 1999b; de Souza et al. 2005b). Under these conditions there is no increase in photoprotection mechanisms or heat energy dissipation (Flexas et al. 2002a; Medrano et al. 2002a) and there is no effect on parameters reflecting photosynthetic capacity, such as Fv/Fm (Flexas et al. 1998; de Souza et al. 2003, 2005b), ETR (Düring 1998; de Souza et al. 2003, 2005b) or the apparent maximum capacity of carboxylation (Vc,max_Ci) or for electron transport (Jmax_Ci) both derived from AN-Ci curves (de Souza et al. 2003). There is also no inhibition of photosynthetic enzymes such as Ribulose-1,5-biphosphate carboxylase/oxygenase (Rubisco) (Bota et al. 2004b), glyceraldehydes-3-phosphate dehydrogenase (G3PDH), ribulose-5-phosphatase kinase (Ru5PK) or fructose-1,6-biphosphate phosphatase (FruBPase) (de Souza et al. 2005b). Therefore, at this stage, diffusional limitations are the only cause for decreased photosynthesis. These are mostly due to stomatal closure, although if the stage is prolonged, mesophyll conductance to CO2 also starts declining, contributing to restricted CO2 diffusion (Flexas et al. 2002a; Moutinho-Pereira et al. 2004), particularly under conditions of high irradiance and temperature (Flexas et al. 2009).
Stage 2 represents a transition phase between predominant stomatal to non-stomatal limitations, and occurs invariably when gs ranges between 50 and 150 mmol H2O m–2 s–1. During this phase a further reduction in AN occurs and WUE usually increases to reach maximum levels, and Ci decreases to minimum levels (Flexas et al. 2002a; Maroco et al. 2002). This indicates that stomatal limitations still dominate, although in some cultivars WUE starts decreasing at this stage, indicating predominant non-stomatal limitations (e.g. Naor et al. 1994). For instance, Vc,max_Ci decreases sometimes, suggesting impairment of Rubisco, which has been shown to decrease its activity (along with activities of G3PDH, Ru5PK and FruBPase) at this stage in some experiments (Maroco et al. 2002). In other experiments, however, Rubisco activity is unaffected (Bota et al. 2004b) and decreases of Vc,max_Ci are due to decreased gm, since when it is calculated on a chloroplast CO2 (Cc) rather than a Ci basis (Vc,max_Cc) it remains unaffected by stress (Flexas et al. 2002a). Fv/Fm remains unaffected (Flexas et al. 1998). There is also no net feedback inhibition of photosynthesis by sugar accumulation (Maroco et al. 2002). Instead, ETR characteristically declines during this phase (Flexas et al. 1999a, 1999b, 2002b), concomitant with increased NPQ, decreased PRI and decreased steady-state chlorophyll fluorescence (Fs) under high light (Flexas et al. 2000; Medrano et al. 2002a; Evain et al. 2004). These changes do not represent permanent damage to photosystems, but rather, they are dynamic and reverse immediately upon shading, e.g. during the passage of a cloud (Evain et al. 2004). As a consequence of both diffusional (i.e. reduced CO2 availability) and biochemical (i.e. impaired Rubisco, decreased ETR) limitations, the rate of photorespiration often declines at this stage back to values similar to those of non-stressed plants (Guan et al. 2004).
Stage 3, when gs drops below 50 mmol H2O m–2 s–1, results in more generalised and dominant non-stomatal limitations to photosynthesis, particularly under conditions where water stress is accompanied by very high temperature and irradiance. This is demonstrated by the fact that photosynthesis cannot be restored even using very high CO2 concentrations (Quick et al. 1992; Flexas et al. 1999b), and there is a drought-induced depression of gross oxygen evolution as seen using O2 isotope discrimination in a mass spectrometer (Flexas et al. 1999b). At this stage, steeper reductions of AN, ETR and Vc,max_Ci occur and NPQ further increases. During this phase AN/gs often decreases and Ci increases steeply (Düring 1987; Flexas et al. 2002a; Sivilotti et al. 2005), indicating that non-stomatal limitations to photosynthesis become dominant. Nevertheless, this is not always observed (see e.g. Fig. 2d). The rate of photorespiration is decreased, but the ratio of photorespiration to photosynthesis still increases, maintaining ETR relatively high with respect to AN (Flexas et al. 1999a, 1999b; de Souza et al. 2005b). Under these conditions, changes in the expression of genes and proteins associated with stomatal functioning and photosynthesis occur (Cramer et al. 2007; Vincent et al. 2007).
Even under these conditions, Fv/Fm is extremely resistant to water stress (Gamon and Pearcy 1990; Flexas et al. 1998). Bertamini et al. (2007) showed that severe water stress does not affect the concentration of the D1 protein and reduces only slightly the electron transport capacities of different components of the electron transport chain. Also, decreases of Vc,max_Ci are sometimes due only to decreased gm (Flexas et al. 2002a) and to errors in the calculation of Ci, as demonstrated by constant Vc,max_Cc. The errors in the calculation of Ci are due to the interference of heterogeneous stomatal closure, which has been shown to occur in grapevine leaves fed with ABA (Downton et al. 1988a) or subject to water stress (Downton et al. 1988b) or salinity (Downton et al. 1990). These errors become important (>10% error in the calculation of Ci) when average gs drops below 30 mmol H2O m–2 s–1 (Flexas et al. 2002a, 2009).
In many other conditions, however, Rubisco activity is truly impaired (Bota et al. 2004b), mostly due to decreases in its concentration (Bota et al. 2004b; Bertamini et al. 2006) and, to a lesser extent, to decreased activation state (Bota et al. 2004b). Moreover, in some cultivars there is a large decrease in the capacity for photoassimilate export out of the leaves (Bota et al. 2004a) resulting in accumulation of soluble sugars in leaves, which can induce feedback inhibition of photosynthesis. In some other cultivars and/or conditions, however, the decrease in the capacity for photoassimilate export is minor and sugars do not accumulate as a consequence of severe water stress (Quick et al. 1992).
Recovery of photosynthesis after water stress
The carbon balance of a plant during a period of water stress and recovery may depend as much on the velocity and degree of photosynthetic recovery as on the degree and velocity of photosynthesis decline during water depletion (Flexas et al. 2006). Relatively few studies have addressed the rate and limiting factors for the recovery of grapevine leaves after water stress. Still, there are some indications suggesting that previous water stress intensity is a crucial factor affecting both the velocity and the extent of recovery after re-watering. For instance, Tempranillo grapevines subjected to ‘Stage 2’ water stress (i.e. maximum stomatal conductance among 0.1–0.15 mol H2O m–2 s–1) recovered completely overnight after re-watering, but ‘Stage 3’ plants of the same cultivar recovered only slowly during the next week, and did not reach the maximum photosynthesis rates reached before water stress (Flexas et al. 2009).
In addition to stress severity, differences associated with both cultivar and environmental conditions may affect recovery. For instance, in cultivars Airén and Chardonnay, gs and AN recovered completely but slowly (3–5 days to complete recovery) after a ‘Stage 2’ water stress. Plants of the rootstock Richter-110 recovered slowly (2 weeks for complete recovery) even after a ‘Stage 1’ water stress (Pou et al. 2008; Flexas et al. 2009). In contrast, several cultivars and rootstocks including Cabernet Sauvignon showed almost complete recovery 2 days after rewatering of ‘Stage 3’ water stressed plants (Guan et al. 2004), similar to ‘Stage 3’ V. labruscana (Liu et al. 1978). Furthermore, gm, Rubisco and ETR recover quickly (1–3 days) after re-watering, although gs remains lower for longer, becoming the most limiting factor for photosynthesis recovery (Flexas et al. 2009).
The reasons for sustained low gs are unclear. Both in cases of rapid recovery of photosynthesis and those in which photosynthesis lasts few days after re-watering, leaf water relations recover fast after re-watering, being totally reversed the day after (Lovisolo et al. 2008a; Pou et al. 2008). In contrast, free cis-trans ABA also recovers control values quickly (1–3 days after re-watering), although hydrolisable cis-trans ABA and phaseic acid remain high for longer (Liu et al. 1978; Pou et al. 2008). Hydraulic conductivity recovers quickly (1 day) in some experiments (Lovisolo and Schubert 2006) but slowly in others (Pou et al. 2008). Lovisolo et al. (2008a) proposed that ABA-induced gs decrease allows embolism repair during the day after re-watering, but this mechanism may not work in cases where ABA is fully reversed in few days while conductivity and gs are not (Pou et al. 2008). Clearly more studies are required to understand the dynamics and mechanisms involved in recovery of grapevine cultivars after water stress.
Cross-talk between water stress responses and berry growth and ripening processes
Following historical linkages between research on grapevine physiology and viticulture techniques, there is a wide and heterogeneous literature related to drought effects on grape berry development, as reviewed by Ollat et al. (2002) and Keller (2005). Knowledge of the phenomenon focuses on (i) effects of an impaired plant metabolism (especially photosynthesis and transpiration) on the accumulation of sugars and secondary metabolites in berry, (ii) consequences at the berry level of both the chemically-mediated long distance signalling between root and shoot (essentially ABA and cytokinin) and the whole-plant hydraulic control via both the xylem and the phloem from root to berry and (iii) berry metabolism adaptations to severe osmotic stress in berry cells.
Water influx into fruits occurs through xylem and phloem and during ripening water flow via the xylem markedly decreases from the onset of véraison when the main source of water to berries becomes the phloem sap. Evidence for ceased xylem functionality in berries has been reported from studies of apoplastic dye perfusion through the pedicel (Greenspan et al. 1996; Rogiers et al. 2001; Bondada et al. 2005; Keller et al. 2006; Tilbrook and Tyerman 2009) even though some studies have been doubtful about the validity of the interpretation of dye uptake experiments (Tyerman et al. 2004). Nevertheless, the hydraulic isolation of the berry due to development of a xylem discontinuity in the pedicel or inside the berry has been considered a way to prevent the loss of solutes in berries (Sarry et al. 2004). Keller et al. (2006) have demonstrated that berry xylem functionality is retained during ripening; the detected decline in xylem water influx into ripening grape berries is due to the apoplastic phloem unloading coupled with solute accumulation in the berry apoplast. More recently, Tilbrook and Tyerman (2009) showed that, according to varietal differences, the activity of semi permeable membranes in mesocarp cells is the key controller of flow in to and out of the berry, dictated by xylem flow. As drought lowers leaf water potential by concentrating osmolites in leaf, drought effects drive water flows between leaf and berry.
Drought effects on accumulation of secondary metabolites in berry were thoroughly investigated since the 1980s. Several studies have measured the effects of water stress on polyphenols (Kennedy et al. 2002; Ojeda et al. 2002; Koundouras et al. 2006; Pedreira dos Santos et al. 2007; Poni et al. 2007) and flavors (Oliveira et al. 2003; Koundouras et al. 2006; Bindon et al. 2007; Pedreira dos Santos et al. 2007). Matthews and Anderson (1988) stated that in the anisohydric Cabernet Franc berry, skin polyphenol and anthocyanin concentrations increased as a consequence of berry volume reduction due to water stress, even though, also expressing data on the basis of surface area, an increase in polyphenol concentration was detected. Roby et al. (2004) found that the concentration of polyphenols such as anthocyanins and proanthocyanidins of berry skins increased after water stress conditions independently from the differences in berry size due to water availability. There was still uncertainty in whether the increases in skin anthocyanins and proanthocyanidins resulted from a differential growth of exocarp cells compared with pulp cells rather than direct effects on phenolic biosynthesis. Recent researches on anisohydric Cabernet Sauvignon (Castellarin et al. 2007a) showed that the expression of some enzymes of the phenylpropoanoid biosynthetic pathway increased as a consequence of early and late water stress application, and also, that this increase was more effective on the biosynthesis of tri-hydroxylated anthocyanins. Early applied water stress was also proved to accelerate the accumulation of sugar and the onset of ripening.
Consequences of chemically(ABA)-mediated signalling and the hydraulic control between root and shoot at the berry level
ABA production in drying roots and its translocation and accumulation in the shoot drive several physiological mechanisms in grapevine leaves (Lovisolo et al. 2002a, 2008a), as reviewed above. In grape berries, ABA is considered a promoter of ripening as its concentration increases at the beginning of véraison (Gagné et al. 2006; Wheeler et al. 2009). Exogenous ABA, used to promote ripening in berries (Peppi et al. 2006; Cantín et al. 2007; Peppi and Fidelibus 2008) induces the accumulation of the mRNA of VvmybA1, a regulatory gene of anthocyanin biosynthesis of grape, leading to an increase of anthocyanin accumulation in Cabernet Sauvignon berry skins (Jeong et al. 2004). ABA also activates invertases (Pan et al. 2005) and VvHT1 (V. vinifera hexose transporter 1) (Çakir et al. 2003). The effect of ABA on UFGT (UDPglucose:flavonoid 3-O-glucosiltransferase), a key-enzyme of the flavonoid biosynthesis, has recently been proved, as well as its influence on the quality of color, the skins of ABA treated Crimson Seedless berries having lower lightness and hue than ABA-untreated control plants (Peppi et al. 2008).
ABA application has little or no effect on berry sugar content at harvest (Jeong et al. 2004; Peppi et al. 2006). The accumulation of sugars in berries requires the coordinated expression of sucrose transporters, invertases, and monosaccharide transporters. The expression of the glucose transporter homologue (VvHT1, V. vinifera hexose transporter 1), isolated from grape berries at véraison, is regulated by sugars and ABA (Vignault et al. 2005). Phloem influx into the berry is accompanied by a decrease in cell turgor (Thomas et al. 2006), which influences the expression of many genes at the onset of ripening (Deluc et al. 2006). Water stress, lowering cell turgor, particularly if applied pre-véraison (Thomas et al. 2006), may induce an increase in sugar influx and ABA, influencing several key steps of the phenylpropanoid biosynthetic pathway. In particular, the expression of genes F3H, F3′5’H, LDOX and DFR involved in the biosynthesis of anthocyanins, proanthocyanidins and flavonols, increases in water-deficit conditions (Castellarin et al. 2007a, 2007b). The increase in total anthocyanins results predominantly from an increase of the F3′5’H expression, responsible for the biosynthesis of tri-hydroxylated anthocyanins. During ripening, the cumulative expression of genes strictly associated to the anthocyanin accumulation UFGT and GST (the latter probably playing a role in the transport of anthocyanins into the vacuole (Ageorges et al. 2006)), is strongly upregulated under water stress conditions (Castellarin et al. 2007a).
The ripening process per se is not accelerated by water deficit in Cabernet Sauvignon or Merlot, since no changes in timing of berry growth and sugar accumulation occur after water deficit (Castellarin et al. 2007a). Furthermore, no earlier downregulation of the genes responsible for proanthocyanidin biosynthesis, which is expected when an early ripening occurs, is detected when water deficit is applied (Castellarin et al. 2007b). The effects of early or late water stress on proanthocyanidin and flavonol concentration and on the expression of genes responsible for their synthesis are still unclear.
Myb transcription factors MybA and Myb5a are higher in water-stressed vines respect to control vines in Merlot, as well as MybC which is upregulated from post-véraison to harvest; MybB and MybD are not influenced by water availability (Castellarin et al. 2007b). In conditions where the accumulation of ABA is higher, the m-RNAs of VvmybA1 are also higher (Yamane et al. 2006); these results suggest that the endogenous ABA level affects the expression of VvmybA1 that controls the expression of the anthocyanin biosynthetic enzyme genes. In Merlot berry skins, the genes NCED1 and NCED2 are only transiently upregulated (Castellarin et al. 2007b) at the onset of véraison after water stress. Moreover, ACPK1 and rd22, both correlated with the ABA metabolism, the former in grape berries (Yu et al. 2006), the latter in other species (Iwasaki et al. 1995), are not at all or only partially upregulated in the experiments reported by Castellarin et al. (2007a, 2007b).
The root and shoot ABA-mediated responses to water stress conditions, or, more generally, to abiotic stresses, are relevant to vine productivity and yield. As described earlier, water stress influences ABA accumulation at the root, shoot and leaf level, and also affects berry quality (Keller 2005). However, a connection between ABA and berry quality has not yet been clarified.
Cytokinin signalling downregulation in ripening berries may represent another point of cross-talk between water stress responses and developmentally-driven ripening processes. Cytokinin concentration sharply decreases at the onset of ripening (Alleweldt et al. 1975), and a zeatin O-glucosyltransferase (Zhang et al. 2008), as well as a gene encoding a cytokinin synthase (Carra et al. 2009), are downregulated in mature berries. Since the repression of cytokinin metabolism is a known response to water deficit in grapevine shoots (Stoll et al. 2000), the relative abundance of ABA and cytokinin may be regulated in the berries by water stress-like signals, and may be important for the coordination of the ripening process. However, direct evidence of drought-induced repression of cytokinin metabolism in berries is still lacking.
High sugar accumulation in ripening grapes generates a severe osmotic stress in berry cells and is accompanied by the expression of thaumatin-like proteins similar to osmotins (Tattersall et al. 1997; Salzman et al. 1998; Davies and Robinson 2000). Osmotins are induced by ABA, thus, it could be that ABA and high sugar concentration may interact to trigger in the berry a defence response, which may also be triggered by water stress. Supporting this idea, transcripts of genes associated with responses to pathogens were shown to be significantly upregulated in the skin of water-stressed plants compared with well watered controls (Grimplet et al. 2007). ABA and sugar signals converge in the induction of VvMSA, an ASR (ABA-, stress- and ripening-induced) protein that acts as a transcriptional activator of the hexose transporter VvHT1 (Çakir et al. 2003). ASR proteins are also induced by drought and salt stress, further supporting the idea that processes characterising berry development and ripening overlap with water stress responses.
Mature grape berries accumulate high amounts of free proline, a well known compatible osmolyte that accumulates in tissues of water stressed plants. Although accumulation of free proline is likely to exert a protective action on grape cellular processes, the molecular determinants of proline homeostasis in berries are largely unknown. In most plant species the reciprocal rates of proline biosynthesis and catabolism are controlled by transcriptional regulation of the rate limiting biosynthetic enzyme Δ1-pyrroline-5-carboxylate synthetase (P5CS) and the catabolic enzyme proline dehydrogenase (PDH) (Verbruggen and Hermans 2008). In grape berries, however, the expression of P5CS and PDH remains relatively unchanged along ripening (Stines et al. 1999; Deluc et al. 2006), despite the rise in concentration of ABA, which is required for the expression of P5CS genes in Arabidopsis (Strizhov et al. 1997). By contrast, in water stressed grapevine, shoot and berry increase in free proline is accompanied by upregulation of both P5CS and PDH (Cramer et al. 2007; Deluc et al. 2009), as well by upregulation of the key ABA biosynthetic gene NCED and of several ABA-responsive genes. Stines et al. (1999) cloned a single P5CS gene from grapevine, and suggested that its protein product may be regulated post-translationally. However, the publication of the grapevine genome sequence draft evidenced that P5CS may be represented by a family of two or three genes in grapevine (Jaillón et al. 2007). Of two family members included in the Arabidopsis genome, P5CS1 is induced by osmotic stress while P5CS2 is developmentally regulated and is required for embryo vitality (Székely et al. 2008). Thus, a yet uncharacterised member of the P5CS family may be responsible for developmentally regulated proline accumulation in ripening grape berries. Alternatively, proline might be translocated to the berry through the phloem.
Partial root-zone drying (PRD): an agronomical application of physiological theory
Knowledge about chemical and hydraulic root signals induced by soil water stress has stimulated new irrigation strategies. Partial root-zone drying (PRD) was developed to improve yield-to-irrigation ratios. PRD is designed to expose part of the root system to drying soil in order to produce the root drought signal, while the remaining roots in wet soil can maintain water supply and, therefore, leaf water potential (Dry and Loveys 1999). PRD enhances root hydraulic conductance in fruit trees; during PRD treatment, roots show higher uptake capacity than in whole root-zone irrigation treatment (Kang et al. 2002). Putative aquaporin stimulation by ABA produced by PRD may be involved. Prolonged exposure of roots to drying soil may cause anatomical changes as epidermis suberisation, collapse of cortex and loss of secondary roots. Alternate watering, after a period of soil drying, may improve this situation by inducing new secondary roots (Kang and Zhang 2004). In the field during a typical drying cycle (10–15 days), only roots near to soil surface feel dry soil whereas deeper roots extract water from wetter soil layers. Maybe this reduces the synthesis of drought chemical signals, like ABA, and, hence, probably reduces PRD effect. Also, the water redistribution process from wet to dry roots (the so-called ‘hydraulic lift’) in response to water potential gradients can contribute to decrease of ABA biosynthesis (De la Hera et al. 2007). This phenomenon has been observed in several grapevines subjected to dry soil conditions or PRD treatment (Smart et al. 2005; Bauerle et al. 2008).
In order to improve PRD technology, further studies may be focused on intra-specific variation in the mechanisms of control of transpiration and their relative sensitivity to soil water deficit, i.e. isohydric and anisohydric behaviour of different grapevine cultivars (Schultz 2003a; Sadras 2009). Genotypically different responses to water stress, such as stomatal sensitivity to non-hydraulic signalling or the ability of the xylem to supply ABA (as described for Grenache and Chardonnay, see above) or rootstock vigour, could be very important factors in determining intensity of PRD response (Antolín et al. 2006; De la Hera et al. 2007). For example, de Souza et al. (2005a) suggest that PRD appears more successful with the more drought-responsive wine grape varieties.
Besides PRD, other techniques aiming at controlling the water balance in vineyards are being adopted in viticultural practice, as discussed by Keller (2005) and Chaves et al. (2007).
Conclusions
We have addressed grapevine responses to water stress by examining perturbations to physiological and molecular processes at the root, shoot, leaf and berry levels (Fig. 3).
Vitis genotypes have been described in relation to their isohydric or anisohydric response to water stress, linked to stomatal behaviour and non-stomatal effects. Stomatal regulation of grapevine is under ABA and hydraulic control; the latter linked to embolism formation and recovery in xylem tissues upstream the stomata. We have focused on ABA effects on stomata and their interrelationship with plant hydraulics from the root towards leaves and with photosynthetic assimilation. Using the daily maximum value of stomatal conductance as an indicator of water stress that allows comparison of plants with iso- and anisohydric behaviours, we have defined three stages of photosynthesis regulation in grapevines subjected to progressive water stress on the basis of the main causes of assimilation decline.
We have shown that in grapevine, xylem embolism occurs and repairs during diurnal cycles under ABA control, and that an almost full recovery of water potential is needed to promote repair mechanisms.
Aquaporins play a fundamental role in the control of plant water status. Different drought-defence strategies between iso- and anisohydric cultivars have been highlighted on the basis of drought-induced root ABA biosynthesis: an increase of suberisation of apoplastic barriers causes a reduction of water conductivity, but this is not compensated by an enhanced aquaporin-mediated cell-to-cell water transport.
Reverse genetics study on key genes of molecular pathways could provide a better understanding of drought tolerance mechanisms in grapevine. The Vitis genus is not usual for genetic transformation (Vivier and Pretorius 2002). However, progress has been recently made in grapevine transformation (Bouquet et al. 2006; Carmona et al. 2008; Burger et al. 2009). Today, a transgenic approach to grapevine improvement is more attractive than a classical breeding approach because a transfer of individual traits as single genes with a minimum disruption of the original genome would leave the traditional characteristics of the cultivar intact (Bouquet et al. 2006).
All aspects reviewed in this paper, when taken together, show that grapevine fits well as a complex, modern model plant for molecular and physiological studies on both plant drought avoidance and tolerance. In particular, knowledge of drought effects on (i) differential development of root and shoot, (ii) long-distance water transport and hydraulic (involving xylem embolism) and hormone signalling, (iii) adaptations of the phosynthetic machinery and of stomatal behaviour and (iv) fruit ripening and technological quality for wine making, highlights the fundamental role that grapevine can play as a model crop. All these aspects are spread on different genotypes either used as rootstocks or as scions, which enlarge the genetic background facing peculiarities known in viticultural practice.
Ageorges A,
Fernandez L,
Vialet S,
Merdinoglu D,
Terrier N, Romieu C
(2006) Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries. Plant Science 170, 372–383.
| Crossref | GoogleScholarGoogle Scholar |

Alleweldt G,
Duering H, Waits G
(1975) Untersuchungen zum Mechanismus der Zuckereinlagerung in die wachsenden Weinbeeren. Angewandte Botanik 49, 65–73.

Alsina MM,
de Herralde F,
Aranda X,
Save R, Biel C
(2007) Water relations and vulnerability to embolism are not related: experiments with eight grapevine cultivars. Vitis 46, 1–6.

Antolín MC,
Ayari M, Sánchez-Díaz M
(2006) Effects of partial rootzone drying on yield, ripening and berry ABA in potted Tempranillo grapevines with split roots. Australian Journal of Grape and Wine Research 12, 13–20.
| Crossref | GoogleScholarGoogle Scholar |

Baiges I,
Schaffner AR, Mas A
(2001) Eight cDNA encoding putative aquaporins in Vitis hybrid Richter-110 and their differential expression. Journal of Experimental Botany 52, 1949–1951.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Baigorri H,
Antolín MC,
De Luis I,
Geny L,
Broquedis M,
Aguirrezábal F, Sánchez-Díaz M
(2001) Influence of training system on the reproductive development and hormonal levels of Vitis vinifera L. cv. Tempranillo. American Journal of Enology and Viticulture 52, 357–363.

Bauerle TL,
Richards JH,
Smart DR, Eissenstat DM
(2008) Importance of internal hydraulic redistribution for prolonging the lifespan of roots in dry soil. Plant, Cell & Environment 31, 177–186.
| PubMed |

Bertamini M, Nedunchezian N
(2004) Photoinhibition and recovery of photosystem 2 in grapevine (Vitis vinifera L.) leaves grown under field conditions. Photosynthetica 41, 611–617.
| Crossref | GoogleScholarGoogle Scholar |

Bertamini M,
Zulini L,
Muthuchelian K, Nedunchezian N
(2006) Effect of water deficit on photosynthetic and other physiological responses in grapevine (Vitis vinifera L. cv. Riesling) plants. Photosynthetica 44, 151–154.
| Crossref | GoogleScholarGoogle Scholar |

Bertamini M,
Zulini L,
Zorer R,
Muthuchelian K, Nedunchezian N
(2007) Photoinhibition of photosynthesis in water deficit leaves of grapevine (Vitis vinifera L.) plants. Photosynthetica 45, 426–432.
| Crossref | GoogleScholarGoogle Scholar |

Bindon KA,
Dry PR, Loveys BR
(2007) Influence of plant water status on the production of C13-norisoprenoid precursors in Vitis vinifera L. cv. Cabernet Sauvignon grape berries. Journal of Agricultural and Food Chemistry 55, 4493–4500.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bisson LF,
Waterhouse AL,
Ebeler SE,
Walker MA, Lapsley JL
(2002) The present and future of the international wine industry. Nature 418, 696–699.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bondada BR,
Matthews MA, Shackel KA
(2005) Functional xylem in the post-veraison grape berry. Journal of Experimental Botany 56(421), 2949–2957.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bota J,
Flexas J, Medrano H
(2001) Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Annals of Applied Biology 138, 353–361.
| Crossref | GoogleScholarGoogle Scholar |

Bota J,
Medrano H, Flexas J
(2004a) Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytologist 162, 671–681.
| Crossref | GoogleScholarGoogle Scholar |

Bota J,
Stasyk O,
Flexas J, Medrano H
(2004b) Effect of water stress on partitioning of 14C-labelled photosynthates in Vitis vinifera. Functional Plant Biology 31, 697–708.
| Crossref | GoogleScholarGoogle Scholar |

Çakir B,
Agasse A,
Gaillard C,
Saumonneau A,
Delrot S, Atanassova R
(2003) A grape ASR protein involved in sugar and abscisic acid signaling. The Plant Cell 15, 2165–2180.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cantín CM,
Fidelibus MW, Crisosto CH
(2007) Application of abscisic acid (ABA) at veraison advanced red color development and maintained postharvest quality of ‘Crimson Seedless’ grapes. Postharvest Biology and Technology 46, 237–241.
| Crossref | GoogleScholarGoogle Scholar |

Carmona MJ,
Chaïb J,
Martínez-Zapater JM, Thomas MR
(2008) A molecular genetic perspective of reproductive development in grapevine. Journal of Experimental Botany 59(10), 2579–2596.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Carra A,
Mica E,
Gambino G,
Pindo M,
Moser C,
Pe ME, Schubert A
(2009) Cloning and characterization of small non-coding RNAs from grape. The Plant Journal 59(5), 750–763.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Castellarin SD,
Matthews MA,
Di Gaspero G, Gambetta GA
(2007a) Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 227, 101–112.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Castellarin SD,
Pfeiffer A,
Sivilotti P,
Degan M,
Peterlunger E, Di Gaspero G
(2007b) Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant, Cell & Environment 30, 1381–1399.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chaumont M,
Morot-Gaudry J-F, Foyer CH
(1994) Seasonal and diurnal changes in photosynthesis and carbon partitioning in Vitis vinifera leaves in vines with and without fruit. Journal of Experimental Botany 45, 1235–1243.
| Crossref | GoogleScholarGoogle Scholar |

Chaumont M,
Morot-Gaudry J-F, Foyer CH
(1995) Effects of photoinhibitory treatment on CO2 assimilation, the quantum yield of CO2 assimilation, D1 protein, ascorbate, glutathione and xanthophyll contents and the electron transport rate in vine leaves. Plant, Cell & Environment 18, 1358–1366.
| Crossref | GoogleScholarGoogle Scholar |

Chaves MM,
Maroco JP, Pereira JS
(2003) Understanding plant responses to drought – from genes to the whole plant. Functional Plant Biology 30, 239–264.
| Crossref | GoogleScholarGoogle Scholar |

Chaves MM,
Santos TP,
Souza CR,
Ortuňo MF,
Rodrigues ML,
Lopes CM,
Maroco JP, Pereira JS
(2007) Deficit irrigation in grapevine improves water use efficiency while controlling vigour and production quality. The Annals of Applied Biology 150, 237–252.
| Crossref | GoogleScholarGoogle Scholar |

Choné X,
van Leeuwen C,
Dubordieu D, Gaudillère JP
(2001) Stem water potential is a sensitive indicator of grapevine water status. Annals of Botany 87, 477–483.
| Crossref | GoogleScholarGoogle Scholar |

Christmann A,
Weiler EW,
Steudle E, Grill E
(2007) A hydraulic signal in root-to-shoot signalling of water shortage. The Plant Journal 52(1), 167–174.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Correia MJ,
Chaves MM, Pereira JS
(1990) Afternoon depression in photosynthesis in grapevine leaves – evidence for a high light stress effect. Journal of Experimental Botany 41, 417–426.
| Crossref | GoogleScholarGoogle Scholar |

Correia MJ,
Pereira JS,
Chaves MM, Pacheco CA
(1995) ABA xylem concentrations determine maximum daily leaf conductance of field-grown Vitis vinifera L. plants. Plant, Cell & Environment 18, 511–521.
| Crossref | GoogleScholarGoogle Scholar |

Cramer GR,
Ergül A,
Grimplet J,
Tillett RL, Tattersall EAR ,
et al
.
(2007) Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Functional & Integrative Genomics 7, 111–134.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cuevas E,
Baeza P, Lissarrague JR
(2006) Variation in stomatal behaviours and gas exchange between mid-morning and mid-afternoon of north-south oriented grapevines (Vitis vinifera L. cv. Tempranillo) at different levels of soil water availability. Scientia Horticulturae 108, 173–180.
| Crossref | GoogleScholarGoogle Scholar |

Davies C, Robinson SP
(2000) Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress response proteins. Plant Physiology 122, 803–812.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

De la Hera ML,
Romero P,
Gomez-Plaza E, Martinez A
(2007) Is partial root-zone drying an effective irrigation technique to improve water use efficiency and fruit quality in field-grown wine grapes under semiarid conditions? Agricultural Water Management 87, 261–274.
| Crossref | GoogleScholarGoogle Scholar |

de Souza CR,
Maroco JP,
dos Santos TP,
Rodrigues ML,
Lopes CM,
Pereira JS, Chaves MM
(2003) Partial root zone drying: regulation of stomatal aperture and carbon assimilation in field-grown grapevines (Vitis vinifera cv. Moscatel). Functional Plant Biology 30, 653–662.
| Crossref | GoogleScholarGoogle Scholar |

de Souza CR,
Maroco JP,
Dos Santos TP,
Rodrigues ML,
Lopes CM,
Pereira JS, Chaves MM
(2005a) Grape berry metabolism in field-grown grapevines exposed to different irrigation strategies. Vitis 44, 103–109.

de Souza CR,
Maroco JP,
dos Santos TP,
Rodrigues ML,
Lopes CM,
Pereira JS, Chaves MM
(2005b) Control of stomatal aperture and carbon uptake by deficit irrigation in two grapevine cultivars. Agriculture Ecosystems & Environment 106, 261–274.
| Crossref | GoogleScholarGoogle Scholar |

Deluc L,
Barrieu F,
Marchive C,
Lauvergeat V,
Decendit A,
Richard T,
Carde JP,
Mérillon JM, Hamdi S
(2006) Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiology 140, 499–511.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Deluc LG,
Quilici DR,
Decendit A,
Grimplet J,
Wheatley MD,
Schlauch KA,
Merillon JM,
Cushman JC, Cramer GR
(2009) Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics 10, 212.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dobrowsky SZ,
Pushnik JC,
Zarco-Tejada PJ, Ustin SL
(2005) Simple reflectance indices track heat and water stress-induced changes in steady-state chlorophyll fluorescence at canopy scale. Remote Sensing of the Environment 97, 403–414.
| Crossref | GoogleScholarGoogle Scholar |

Downton WJS
(1983) Osmotic adjustment during water stress protects the photosynthetic apparatus against photoinhibition. Plant Science Letters 30, 137–143.
| Crossref | GoogleScholarGoogle Scholar |

Downton WJS,
Grant WJR, Loveys BR
(1987) Diurnal changes in the photosynthesis of field-grown grape vines. New Phytologist 105, 71–80.
| Crossref | GoogleScholarGoogle Scholar |

Downton WJS,
Loveys BR, Grant WJR
(1988a) Stomatal closure fully accounts for the inhibition of photosynthesis by abscisic acid. New Phytologist 108, 263–266.
| Crossref | GoogleScholarGoogle Scholar |

Downton WJS,
Loveys BR, Grant WJR
(1988b) Non-uniform stomatal closure induced by water stress causes putative non-stomatal inhibition of photosynthesis. New Phytologist 110, 503–509.
| Crossref | GoogleScholarGoogle Scholar |

Downton WJS,
Loveys BR, Grant WJR
(1990) Salinity effects on the stomatal behaviour of grapevine. New Phytologist 116, 499–503.
| Crossref | GoogleScholarGoogle Scholar |

Dry PR, Loveys BR
(1998) Factors influencing grapevine vigour and the potential for control with partial rootzone drying. Australian Journal of Grape and Wine Research 4, 140–148.
| Crossref | GoogleScholarGoogle Scholar |

Dry PR, Loveys BR
(1999) Grapevine shoot growth and stomatal conductance are reduced when part of the root system is dried. Vitis 38, 151–156.

Dry PR,
Loveys BR, Düring H
(2000a) Partial drying of the rootzone of grape. I. Transient changes in shoot growth and gas exchange. Vitis 39, 3–7.

Dry PR,
Loveys BR, Düring H
(2000b) Partial drying of the rootzone of grape. II. Changes in the pattern of root development. Vitis 39, 9–12.

Düring H
(1984) Evidence for osmotic adjustment to drought in grapevines. Vitis 23, 1–10.

Düring H
(1987) Stomatal responses to alterations of soil and air humidity in grapevines. Vitis 26, 9–18.

Düring H
(1991) Determination of the photosynthetic capacity of grapevines leaves. Vitis 30, 49–56.

Düring H
(1998) Photochemical and non-photochemical responses of glasshouse-grown grape to combined light and water stress. Vitis 37, 1–4.

Düring H
(1999) Photoprotection in leaves of grapevines: responses of the xanthophylls cycle to alterations of light intensity. Vitis 38, 21–24.

Escalona JM,
Flexas J,
Bota J, Medrano H
(2003) From leaf photosynthesis to grape yield: influence of soil water availability. Vitis 42, 57–64.

Evain S,
Flexas J, Moya I
(2004) A new instrument for passive remote sensing: 2. Measurement of leaf and canopy reflectance changes at 531 nm and their relationship with photosynthesis and chlorophyll fluorescence. Remote Sensing of Environment 91, 175–185.
| Crossref | GoogleScholarGoogle Scholar |

Flexas J,
Escalona JM, Medrano H
(1998) Down-regulation of photosynthesis by drought under field conditions in grapevine leaves. Australian Journal of Plant Physiology 25, 893–900.
| Crossref | GoogleScholarGoogle Scholar |

Flexas J,
Escalona JM, Medrano H
(1999a) Water stress induces different photosynthesis and electron transport rate regulation in grapevine. Plant, Cell & Environment 22, 39–48.
| Crossref | GoogleScholarGoogle Scholar |

Flexas J,
Badger M,
Chow WS,
Medrano H, Osmond CB
(1999b) Analysis of the relative increase in photosynthetic O2 uptake when photosynthesis in grapevine leaves is inhibited following low night temperatures and/or water stress. Plant Physiology 121, 675–684.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Flexas J,
Briantais J-M,
Cerovic Z,
Medrano H, Moya I
(2000) Steady-state and maximum chlorophyll fluorescence responses to water stress in grapevine leaves: a new remote sensing system. Remote Sensing of Environment 73, 283–297.
| Crossref | GoogleScholarGoogle Scholar |

Flexas J,
Hendrickson L, Chow WS
(2001) Photoinactivation of photosystem II in high light-acclimated grapevines. Australian Journal of Plant Physiology 28, 755–764.

Flexas J,
Bota J,
Escalona JM,
Sampol B, Medrano H
(2002a) Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology 29, 461–471.
| Crossref | GoogleScholarGoogle Scholar |

Flexas J,
Escalona JM,
Evain S,
Gulías J,
Moya I,
Osmond CB, Medrano H
(2002b) Steady-state chlorophyll fluorescence (F
s) measurements as a tool to follow variations of net CO2 assimilation and stomatal conductance during water-stress in C3 plants. Physiologia Plantarum 114, 231–240.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Flexas J,
Bota J,
Galmés J,
Medrano H, Ribas-Carbó M
(2006) Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiologia Plantarum 127, 343–352.
| Crossref | GoogleScholarGoogle Scholar |

Flexas J,
Ribas-Carbo M,
Diaz-Espejo A,
Galmés J, Medrano H
(2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell & Environment 31, 602–621.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Flexas J,
Barón M,
Bota J,
Ducruet J-M, Gallé A ,
et al
.
(2009) Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). Journal of Experimental Botany 60, 2361–2377.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fouquet R,
Leon C,
Ollat N, Barrieu F
(2008) Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Reports 27(9), 1541–1550.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fromm J, Fei HM
(1998) Electrical signaling and gas exchange in maize plants of drying soil. Plant Science 132(2), 203–213.
| Crossref | GoogleScholarGoogle Scholar |

Froux F,
Ducrey M,
Dreyer E, Huc R
(2005) Vulnerability to embolism differs in roots and shoots and among three Mediterranean conifers: consequences for stomatal regulation of water loss? Trees – Structure and Function 19, 137–144.

Gagné S,
Esteve K,
Deytieux C,
Saucier C, Geny L
(2006) Influence of abscissic acid in triggering ‘véraison’ in grape berry skins of Vitis vinifera L. cv. Cabernet Sauvignon. Journal International des Sciences de la Vigne et du Vin 40, 7–14.

Galmés J,
Pou A,
Alsina MM,
Tomas M,
Medrano H, Flexas J
(2007) Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): relationship with ecophysiological status. Planta 226(3), 671–681.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gamon JA, Pearcy RW
(1990) Photoinhibition in Vitis californica: interactive effects of sunlight, temperature and water status. Plant, Cell & Environment 13, 267–275.
| Crossref | GoogleScholarGoogle Scholar |

Gaudillère JP,
van Leeuwen C, Ollat N
(2002) Carbon isotope composition of sugars in grapevine, and integrated indicator of vineyard water status. Journal of Experimental Botany 53, 757–763.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gibberd MR,
Walker RR,
Blackmore DH, Condon AG
(2001) Transpiration efficiency and carbon-isotope discrimination of grapevines grown under well-watered conditions in either glasshouse or vineyard. Australian Journal of Grape and Wine Research 7, 110–117.
| Crossref | GoogleScholarGoogle Scholar |

Glissant D,
Dedaldechamp F, Delrot S
(2008) Transcriptomic analysis of grape berry softening during ripening. Journal International des Sciences de la Vigne et du Vin 42, 1–13.

Gómez-del-Campo M,
Baeza P,
Ruiz C, Lissarrague JR
(2004) Water-stress induced physiological changes in leaves of four container-grown grapevine cultivars (Vitis vinifera L.). Vitis 43, 99–105.

Grams TEE,
Koziolek C,
Lautner S,
Matyssek R, Fromm J
(2007) Distinct roles of electric and hydraulic signals on the reaction of leaf gas exchange upon re-irrigation in Zea mays L. Plant, Cell & Environment 30, 79–84.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Greenspan MD,
Schultz HR, Matthews MA
(1996) Field evaluation of water transport in grape berries during water deficits. Physiologia Plantarum 97, 55–62.
| Crossref | GoogleScholarGoogle Scholar |

Grimplet J,
Deluc LG,
Tillett RL,
Wheatley MD,
Schlauch KA,
Cramer GR, Cushman JC
(2007) Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics 8, 187.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Guan XQ,
Zhao SJ,
Li DQ, Shu HR
(2004) Photoprotective function of photorespiration in several grapevine cultivars under drought stress. Photosynthetica 42, 31–36.
| Crossref | GoogleScholarGoogle Scholar |

Hardie WJ, Martin SR
(2000) Shoot growth on de-fruited grapevines: a physiological indicator for irrigation scheduling. Australian Journal of Grape and Wine Research 6, 52–58.
| Crossref | GoogleScholarGoogle Scholar |

Hukin D,
Cochard H,
Dreyer E,
Thiec DL, Bogeat-Triboulot MB
(2005) Cavitation vulnerability in roots and shoots: does Populus euphratica Oliv., a poplar from arid areas of Central Asia, differ from other poplar species? Journal of Experimental Botany 56, 2003–2010.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Iacono F, Sommer KJ
(1996) Photoinhibition of photosynthesis and photorespiration in Vitis vinifera under field conditions – effects of light climate and leaf position. Australian Journal of Grape and Wine Research 2, 1–11.
| Crossref | GoogleScholarGoogle Scholar |

Intrigliolo DS, Castel JR
(2006) Vine and soil-based measures of water status in a Tempranillo vineyard. Vitis 45, 157–163.

Ishimaru M,
Smith DL,
Gross KC, Kobayashi S
(2007) Expression of three expansin genes during development and maturation of Kyoho grape berries. Journal of Plant Physiology 164, 1675–1682.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Iwasaki T,
Yamaguchi-Shinozaki K, Shinozaki K
(1995) Identification of a cis-regulatory region of a gene in Arabidopsis thaliana whose induction by dehydration is mediated by abscissic acid and requires protein synthesis. Molecular & General Genetics 247, 391–398.
| Crossref | GoogleScholarGoogle Scholar |

Jaillón O,
Aury JM,
Noel B,
Policriti A, Clepet C ,
et al
.
(2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449, 463–467.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jang JY,
Kim DG,
Kim YO,
Kim JS, Kang H
(2004) An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Molecular Biology 54, 713–725.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jeong ST,
Goto-Yamamoto N,
Kobayashi S, Esaka M
(2004) Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Science 167, 247–252.
| Crossref | GoogleScholarGoogle Scholar |

Johansson I,
Karlsson M,
Johanson U,
Larsson C, Kjellbom P
(2000) The role of aquaporins in cellular and whole plant water balance. Biochimica and Biophysica Acta – Biomembranes 1465, 324–342.
| Crossref | GoogleScholarGoogle Scholar |

Kaldenhoff R,
Ribas-Carbo M,
Flexas J,
Lovisolo C,
Heckwolf M, Uehlein N
(2008) Aquaporins and plant water balance. Plant, Cell & Environment 31, 658–666.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kang S, Zhang J
(2004) Controlled alternate partial root-zone irrigation: its physiological consequences and impact on water use efficiency. Journal of Experimental Botany 55, 2437–2446.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kang SZ,
Hu XT,
Goodwin I,
Jirie P, Zhang J
(2002) Soil water distribution, water use and yield response to partial root-zone drying under flood-irrigation condition in a pear orchard. Scientia Horticulturae 92, 277–291.
| Crossref | GoogleScholarGoogle Scholar |

Keller M
(2005) Deficit irrigation and vine mineral nutrition. American Journal of Enology and Viticulture 56, 267–283.

Keller M,
Smith JP, Bondada BR
(2006) Ripening grape berries remain hydraulically connected to the shoot. Journal of Experimental Botany 57, 2577–2587.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kennedy JA,
Matthews MA, Waterhouse AL
(2002) Effect of maturity and vine water status on grape skins and wine flavonoids. American Journal of Enology and Viticulture 53, 268–274.

Koundouras S,
Marinos V,
Gkoulioti A,
Kotseridis Y, van Leeuwen C
(2006) Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv. Agiorgitiko (Vitis vinifera L.) effects on wine phenolic and aroma components. Journal of Agricultural and Food Chemistry 54, 5077–5086.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lebon E,
Pellegrino A,
Louarn G, Lecoeur J
(2006) Branch development controls leaf area dynamics in grapevine (Vitis vinifera) growing in drying soil. Annals of Botany 98, 175–185.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Liu WT,
Pool R,
Wenkert W, Kriedemann PE
(1978) Changes in photosynthesis, stomatal resistance and abscisic acid of Vitis labruscana through drought and irrigation cycles. American Journal of Enology and Viticulture 29, 239–246.

Loveys BR
(1984a) Abscisic acid transport and metabolism in grapevine (Vitis vinifera L.). New Phytologist 98, 575–582.
| Crossref | GoogleScholarGoogle Scholar |

Loveys BR
(1984b) Diurnal changes in water relations and abscisic acid in field-grown Vitis vinifera cultivars. III. The influence of xylem-derived abscisic acid on leaf gas exchange. New Phytologist 98, 563–573.
| Crossref | GoogleScholarGoogle Scholar |

Loveys BR, Kriedemann PE
(1974) Internal control of stomatal physiology and photosynthesis. I. Stomatal regulation and associated changes in endogenous levels of abscisic and phaseic acids. Australian Journal of Plant Physiology 1, 407–415.
| Crossref | GoogleScholarGoogle Scholar |

Lovisolo C, Schubert A
(1998) Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. Journal of Experimental Botany 49, 693–700.
| Crossref | GoogleScholarGoogle Scholar |

Lovisolo C, Schubert A
(2006) Mercury hinders recovery of shoot hydraulic conductivity during grapevine rehydration: evidence from a whole-plant approach. New Phytologist 172, 469–478.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lovisolo C,
Hartung W, Schubert A
(2002a) Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid are independently affected by water stress in grapevines. Functional Plant Biology 29, 1349–1356.
| Crossref | GoogleScholarGoogle Scholar |

Lovisolo C,
Schubert A, Sorce C
(2002b) Are xylem radial development and hydraulic conductivity in downwardly-growing grapevine shoots influenced by perturbed auxin metabolism? New Phytologist 156, 65–74.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lovisolo C,
Perrone I,
Hartung W, Schubert A
(2008a) An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytologist 180, 642–651.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lovisolo C,
Tramontini S,
Flexas J, Schubert A
(2008b) Mercurial inhibition of root hydraulic conductance in Vitis spp. rootstocks under water stress. Environmental and Experimental Botany 63, 178–182.
| Crossref | GoogleScholarGoogle Scholar |

Lu P,
Yunusa IAM,
Walker RR, Müller WJ
(2003) Regulation of canopy conductance and transpiration and their modelling in irrigated grapevines. Functional Plant Biology 30, 689–698.
| Crossref | GoogleScholarGoogle Scholar |

Mancuso S
(1999) Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Australian Journal of Plant Physiology 26, 55–61.
| Crossref | GoogleScholarGoogle Scholar |

Mapfumo E, Aspinall D
(1994) Anatomical changes of grapevine (Vitis vinifera L. cv. Shiraz) roots related to radial resistance to water movement. Australian Journal of Plant Physiology 21, 437–447.
| Crossref | GoogleScholarGoogle Scholar |

Mapfumo E,
Aspinall D,
Hancock T, Sedgley M
(1993) Xylem development in relation to water uptake by roots of grapevine (Vitis vinifera L.). New Phytologist 125, 93–99.
| Crossref | GoogleScholarGoogle Scholar |

Mariaux JB,
Bockel C,
Salamini F, Bartels D
(1998) Dessication and abscisic acid-responsive genes encoding major intrinsic proteins (MIPs) from the resurrection plant Craterostigma plantagineum. Plant Molecular Biology 38, 1089–1099.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Maroco JP,
Rodríguez ML,
Lopes C, Chaves MM
(2002) Limitations to leaf photosynthesis in field-grown grapevine under drought – metabolic and modelling approaches. Functional Plant Biology 29, 451–459.
| Crossref | GoogleScholarGoogle Scholar |

Martre P,
Morillon R,
Barrieu F,
North GB,
Nobel PS, Chrispeels MJ
(2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiology 130, 2101–2110.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Matthews MA, Anderson MM
(1988) Fruit ripening in Vitis vinifera L.: responses to seasonal water deficits. American Journal of Enology and Viticulture 39, 313–320.

Maurel C,
Verdoucq L,
Luu DT, Santoni V
(2008) Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology 59, 595–624.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Medrano H,
Bota J,
Abadía A,
Sampol B,
Escalona JM, Flexas J
(2002a) Drought effects on light energy dissipation in high light-acclimated, field-grown grapevines. Functional Plant Biology 29, 1197–1207.
| Crossref | GoogleScholarGoogle Scholar |

Medrano H,
Escalona JM,
Bota J,
Gulías J, Flexas J
(2002b) Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany 89, 895–905.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Medrano H,
Escalona JM,
Cifre J,
Bota J, Flexas J
(2003) Regulated deficit irrigation effects in cv. ‘Tempranillo’ vineyards grown under semiarid conditions in mid-Ebro river valley (Spain). Functional Plant Biology 30, 607–619.
| Crossref | GoogleScholarGoogle Scholar |

Morlat R, Jacquet A
(1993) The soil effects on the grapevine root-system in several vineyards of the Loire valley (France). Vitis 32, 35–42.

Moutinho-Pereira JM,
Correia CM,
Gonçalves BM,
Bacelar EA, Torres-Pereira JM
(2004) Leaf gas exchange and water relations of grapevines grown in three different conditions. Photosynthetica 42, 81–86.
| Crossref | GoogleScholarGoogle Scholar |

Naor A, Wample RL
(1994) Gas-exchange and water relations of field-grown Concord (Vitis labruscana Bailey) grapevines. American Journal of Enology and Viticulture 45(3), 333–337.

Naor A,
Bravdo B, Gelobter J
(1994) Gas exchange and water relations in field-grown Sauvignon Blanc grapevines. American Journal of Enology and Viticulture 45, 423–428.

Ojeda H,
Andary C,
Kraeva E,
Carbonneau A, Deloire A
(2002) Influence of pre- and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera cv. Shiraz. American Journal of Enology and Viticulture 53, 261–267.

Oliveira C,
Silva-Ferreira AC,
Mendes Pinto M,
Hogg T,
Alves F, Guedes de Pinho P
(2003) Carotenoid compounds in grapes and their relationship to plant water status. Journal of Agricultural and Food Chemistry 51, 5967–5971.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ollat N,
Diakou-Verdin P,
Carde JP,
Barrieu F,
Gaudillere JP, Moing A
(2002) Grape berry development: a review. Journal International des Sciences de la Vigne et du Vin 36, 109–131.

Padgett-Johnson M,
Williams LE, Walker MA
(2000) The influence of Vitis riparia rootstock on water relations and gas exchange of Vitis vinifera cv. Carignane scion under non-irrigated conditions. American Journal of Enology and Viticulture 51, 137–143.

Pan QH,
Li MJ,
Peng CC,
Zhang N,
Zou X,
Zou KQ,
Wang XL,
Yu XC,
Wang XF, Zhang DP
(2005) Abscisic acid activates acid invertases in developing grape berry. Physiologia Plantarum 125, 157–170.
| Crossref | GoogleScholarGoogle Scholar |

Parent B,
Hachez C,
Redondo E,
Simonneau T,
Chaumont F, Tardieu F
(2009) Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiology 149, 2000–2012.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Patakas A, Noitsakis B
(1997) Cell wall elasticity as a mechanism to maintain favourable water relations during leaf ontogeny in grapevines. American Journal of Enology and Viticulture 48, 352–356.

Patakas A,
Noitsakis B, Chouzouri A
(2005) Optimization of irrigation water use in grapevines using the relationship between transpiration and plant water status. Agriculture Ecosystems & Environment 106, 253–259.
| Crossref | GoogleScholarGoogle Scholar |

Pedreira dos Santos T,
Lopes CM,
Rodrigues ML,
de Souza CR,
Ricardo-da-Silva JM,
Maroco JP,
Pereira JS, Chaves MM
(2007) Effect of deficit irrigation strategies on cluster microclimate for improving composition of Moscatel field-grown grapevines. Scientia Horticulturae 112, 321–330.
| Crossref | GoogleScholarGoogle Scholar |

Pellegrino A,
Lebon E,
Simmoneau T, Wery J
(2005) Towards a simple indicator of water stress in grapevine (Vitis vinifera L.) based on the differential sensitivities of vegetative growth components. Australian Journal of Grape and Wine Research 11, 306–315.
| Crossref | GoogleScholarGoogle Scholar |

Peppi MC, Fidelibus MW
(2008) Effects of forchlorfenuron and abscisic acid on the quality of ‘Flame Seedless’ grapes. HortScience 43, 173–176.

Peppi MC,
Fidelibus MW, Dokoozlian N
(2006) Abscisic acid application timing and concentration affect firmness, pigmentation, and color of ‘Flame Seedless’ grapes. HortScience 41, 1440–1445.

Peppi MC,
Walker MA, Fidelibus MW
(2008) Application of abscisic acid rapidly upregulated UFGT gene expression and improved color of grape berries. Vitis 47, 11–14.

Picaud S,
Becq F,
Dedaldechamp F,
Ageorges A, Delrot S
(2003) Cloning and expression of two plasma membrane aquaporins expressed during the ripening of grape berry. Functional Plant Biology 30, 621–630.
| Crossref | GoogleScholarGoogle Scholar |

Poni S,
Lakso AN,
Turner JR, Melious RE
(1993) The effects of pre- and post-veraison water stress on growth and physiology of potted Pinot Noir grapevines at varying crop levels. Vitis 32, 207–214.

Poni S,
Bernizzoni F, Civardi S
(2007) Response of ‘Sangiovese’ grapevines to partial root-zone drying: gas-exchange, growth and grape composition. Scientia Horticulturae 114, 96–103.
| Crossref | GoogleScholarGoogle Scholar |

Pou A,
Flexas J,
Alsina MM,
Bota J, Carambula C ,
et al
.
(2008) Adjustments of water-use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). Physiologia Plantarum 134, 313–323.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Quereix A,
Dewar RC,
Gaudillere J-P,
Dayau S, Valancogne C
(2001) Sink feedback regulation of photosynthesis in vines: measurements and a model. Journal of Experimental Botany 52, 2313–2322.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Quick WP,
Chaves MM,
Wendler R,
David M,
Rodrigues ML,
Passaharinho JA,
Pereira JS,
Adcock MD,
Leegood RC, Stitt M
(1992) The effect of water stress on photosynthetic carbon metabolism in four species grown under field conditions. Plant, Cell & Environment 15, 25–35.
| Crossref | GoogleScholarGoogle Scholar |

Reid KE,
Olsson N,
Schlosser J,
Peng F, Lund ST
(2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biology 6, 27.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Roby G,
Harbertson JF,
Adams DA, Matthews MA
(2004) Berry size and vine water deficits as factors in winegrape composition: anthocyanins and tannins. Australian Journal of Grape and Wine Research 10, 100–107.
| Crossref | GoogleScholarGoogle Scholar |

Rodrigues ML,
Chaves MM,
Wendler R,
David MM,
Quick WP,
Leegood RC,
Stitt M, Pereira JS
(1993) Osmotic adjustment in water stressed grapevine leaves in relation to carbon assimilation. Australian Journal of Plant Physiology 20, 309–321.
| Crossref | GoogleScholarGoogle Scholar |

Rodrigues ML,
Santos TP,
Rodrigues AP,
de Souza CR,
Lopes CM,
Maroco JP,
Pereira JS, Chaves MM
(2008) Hydraulic and chemical signalling in the regulation of stomatal conductance and plant water use in field grapevines growing under deficit irrigation. Functional Plant Biology 35, 565–579.
| Crossref | GoogleScholarGoogle Scholar |

Rogiers SY,
Smith JA,
White R,
Keller M,
Holzapfel BP, Virgona JM
(2001) Vascular function in berries of Vitis vinifera (L) cv. Shiraz. Australian Journal of Grape and Wine Research 7, 47–51.
| Crossref | GoogleScholarGoogle Scholar |

Sadras VO
(2009) Does partial root-zone drying improve irrigation water productivity in the field? A meta-analysis. Irrigation Science 27, 183–190.
| Crossref | GoogleScholarGoogle Scholar |

Sakr S,
Alves G,
Morillon R,
Maurel K,
Decourteix M,
Guilliot A,
Fleurat-Lessard P,
Julien JL, Chrispeels MJ
(2003) Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree. Plant Physiology 133, 630–641.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Salleo S,
Rosso R, Lo Gullo MA
(1982) Hydraulic architecture of Vitis vinifera L. and Populus deltoides Bartr. 1-year-old twigs: I – hydraulic conductivity (LSC) and water potential gradients. Giornale Botanico Italiano (Florence, Italy) 116, 15–27.

Salleo S,
Lo Gullo MA, Oliveri F
(1985) Hydraulic parameters measured in 1-year-old twigs of some Mediterranean species with diffuse-porous wood: changes in hydraulic conductivity and their possible functional significance. Journal of Experimental Botany 36, 1–11.
| Crossref | GoogleScholarGoogle Scholar |

Salleo S,
Nardini A,
Pitt F, Lo Gullo MA
(2000) Xylem cavitation and hydraulic control of stomatal conductance in laurels (Laurus nobilis L.). Plant, Cell & Environment 23, 71–79.
| Crossref | GoogleScholarGoogle Scholar |

Salleo S,
Lo Gullo MA,
Trifilo P, Nardini A
(2004) New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant, Cell & Environment 27, 1065–1076.
| Crossref | GoogleScholarGoogle Scholar |

Salzman RA,
Tikhonova I,
Bordelon BP,
Hasegawa PM, Bressan RA
(1998) Coordinate accumulation of antifungal proteins and hexoses constitutes a developmentally controlled defense response during fruit ripening in grape. Plant Physiology 117, 465–472.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sarry JE,
Sommerer N,
Sauvage FX,
Bergoin A,
Rossignol M,
Albagnac G, Romieu C
(2004) Grape berry biochemistry revisited upon proteomic analysis of the mesocarp. Proteomics 4, 201–215.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Satisha J,
Prakash GS, Venugopalan R
(2006) Statistical modeling of the effect zof physio-biochemical parameters on water use efficiency of grape varieties, rootstocks and their stionic combinations under moisture stress conditions. Turkish Journal of Agriculture and Forestry 30, 261–271.

Schlosser J,
Olsson N,
Weis M,
Reid K,
Peng F,
Lund S, Bowen P
(2008) Cellular expansion and gene expression in the developing grape (Vitis vinifera L.). Protoplasma 232, 255–265.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schultz HR
(2003a) Differences in hydraulic architecture account for near isohydric and anisohydric behaviours of two field-grown Vitis vinifera L. cultivars during drought. Plant, Cell & Environment 26, 1393–1405.
| Crossref | GoogleScholarGoogle Scholar |

Schultz HR
(2003b) Extension of a Farquhar model for limitations of leaf photosynthesis induced by light environment, phenology and leaf age in grapevines (Vitis vinifera L. cvv. White Riesling and Zinfandel). Functional Plant Biology 30, 673–687.
| Crossref | GoogleScholarGoogle Scholar |

Schultz HR, Matthews MA
(1988) Resistance to water transport in shoots of Vitis vinifera L.: relation to growth at low water potential. Plant Physiology 88, 718–724.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schultz HR, Matthews MA
(1993) Growth, osmotic adjustment, and cell-wall mechanics of expanding grape leaves during water deficits. Crop Science 33, 287–294.

Shelden MC,
Howitt SM,
Kaiser BN, Tyerman SD
(2009) Identification and functional characterisation of aquaporins in the grapevine, Vitis vinifera. Functional Plant Biology 36, 1065–1078.
| Crossref | GoogleScholarGoogle Scholar |

Sivilotti P,
Bonetto C,
Paladin M, Peterlunger E
(2005) Effect of soil moisture availability on Merlot: from leaf water potential to grape composition. American Journal of Enology and Viticulture 56, 9–18.

Smart DR,
Carlisle E,
Goebel M, Nunez BA
(2005) Transverse hydraulic redistribution by a grapevine. Plant, Cell & Environment 28, 157–166.
| Crossref | GoogleScholarGoogle Scholar |

Soar CJ,
Speirs J,
Maffei SM, Loveys BR
(2004) Gradients in stomatal conductance, xylem sap ABA and bulk leaf ABA along canes of Vitis vinifera cv. Shiraz: molecular and physiological studies investigating their source. Functional Plant Biology 31, 659–669.
| Crossref | GoogleScholarGoogle Scholar |

Soar CJ,
Speirs J,
Maffei SM,
Penrose AB,
McCarthy MG, Loveys BR
(2006) Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Australian Journal of Grape and Wine Research 12, 2–12.
| Crossref | GoogleScholarGoogle Scholar |

Sperry JS,
Hacke UG, Pittermann J
(2006) Size and function in conifer tracheids and angiosperm vessels. American Journal of Botany 93, 1490–1500.
| Crossref | GoogleScholarGoogle Scholar |

Stevens RM,
Harvey G, Aspinall D
(1995) Grapevine growth of shoots and fruit linearly correlate with water stress indices based on root-weighted soil matric potential. Australian Journal of Grape and Wine Research 1, 58–66.
| Crossref | GoogleScholarGoogle Scholar |

Stines AP,
Naylor DJ,
Høj PB, van Heeswijck R
(1999) Proline accumulation in developing grapevine fruit occurs independently of changes in the levels of 1-pyrroline-5-carboxylate synthetase mRNA or protein. Plant Physiology 120, 923–931.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Stoll M,
Loveys BR, Dry P
(2000) Hormonal changes induced by partial rootzone drying of irrigated grapevine. Journal of Experimental Botany 51, 1627–1634.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Strizhov N,
Abraham E,
Okresz L,
Blickling S,
Zilberstein A,
Schell J,
Koncz C, Szabados L
(1997) Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. The Plant Journal 12, 557–569.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Suga S,
Komatsu S, Maeshima M
(2002) Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant & Cell Physiology 43, 1229–1237.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Székely G,
Abrahám E,
Cséplo A,
Rigó G, Zsigmond L ,
et al
.
(2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. The Plant Journal 53, 11–28.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tattersall DB,
van Heeswijck R, Hoj PB
(1997) Identification and characterization of a fruit-specific, thaumatin-like protein that accumulates at very high levels in conjunction with the onset of sugar accumulation and berry softening in grapes. Plant Physiology 114, 759–769.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Thomas P,
Lee MM, Schiefelbein J
(2003) Molecular identification of proline-rich protein genes induced during root formation in grape (Vitis vinifera L.) stem cuttings. Plant, Cell & Environment 26, 1497–1504.
| Crossref | GoogleScholarGoogle Scholar |

Thomas TR,
Matthews MA, Shackel K
(2006) Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant, Cell & Environment 29, 993–1001.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tilbrook J, Tyerman SD
(2009) Hydraulic connection of grape berries to the vine: varietal differences in water conductance into and out of berries, and potential for backflow. Functional Plant Biology 36, 541–550.
| Crossref | GoogleScholarGoogle Scholar |

Troggio M,
Vezzulli S,
Pindo M,
Malacarne G,
Fontana P,
Moreira FM,
Costantini L,
Grando MS,
Viola R, Velasco R
(2008) Beyond the genome, opportunities for a modern viticulture: a research overview. American Journal of Enology and Viticulture 59, 117–127.

Tyerman SD,
Tilbrook J,
Pardo C,
Kotula L,
Sullivan W, Steudle E
(2004) Direct measurement of hydraulic properties in developing berries of Vitis vinifera L. cvs Shiraz and Chardonnay. Australian Journal of Grape and Wine Research 10, 170–181.

Vandeleur RK,
Mayo G,
Shelden MC,
Gilliham M,
Kaiser BN, Tyerman SD
(2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiology 149, 445–460.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vera-Estrella R,
Barkla BJ,
Bohnert HJ, Pantoja O
(2004) Novel regulation of aquaporins during osmotic stress. Plant Physiology 135, 2318–2329.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Verbruggen N, Hermans C
(2008) Proline accumulation in plants: a review. Amino Acids 35, 753–759.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vignault C,
Vachaud M,
Çakir B,
Glissant D,
Dédaldéchamp F,
Büttner M,
Atanassova R,
Fleurat-Lessard P,
Lemoine R, Delrot S
(2005)
VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. Journal of Experimental Botany 56, 1409–1418.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vincent D,
Ergül A,
Bohlman MC,
Tattersall EAR, Tillett RL ,
et al
.
(2007) Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet Sauvignon and their responses to water deficit and salinity. Journal of Experimental Botany 58, 1873–1892.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vivier MA, Pretorius IS
(2002) Genetically tailored grapevines for the wine industry. Trends in Biotechnology 20, 472–478.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wan XC,
Steudle E, Hartung W
(2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. Journal of Experimental Botany 55, 411–422.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wheeler S,
Loveys B,
Ford C, Davies C
(2009) The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Australian Journal of Grape and Wine Research 15, 195–204.
| Crossref | GoogleScholarGoogle Scholar |

Williams LE, Baeza P
(2007) Relationships among ambient temperature and vapor pressure deficit and leaf and stem water potentials of fully irrigated, field-grown grapevines. American Journal of Enology and Viticulture 58(2), 173–181.

Winkel T, Rambal S
(1990) Stomatal conductance of some grapevines growing in the field under a Mediterranean environment. Agricultural and Forest Meteorology 51, 107–121.
| Crossref | GoogleScholarGoogle Scholar |

Winkel T, Rambal S
(1993) Influence of water stress on grapevines growing in the field: from leaf to whole-plant response. Australian Journal of Plant Physiology 20, 143–157.
| Crossref | GoogleScholarGoogle Scholar |

Yamane T,
Jeong ST,
Goto-Yamamoto N,
Koshita Y, Kobayashi S
(2006) Effects of temperature on anthocyanin biosynthesis in grape berry skins. American Journal of Enology and Viticulture 57, 54–59.

Yu XC,
Li MJ,
Gao GF,
Feng HZ,
Geng XQ,
Peng CC,
Zhu SY,
Wang XJ,
Shen YY, Zhang DP
(2006) Abscisic acid stimulates a calcium-dependent protein kinase in grape berry. Plant Physiology 140, 558–579.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhang JW,
Ma HQ,
Feng JD,
Zeng L,
Wang Z, Chen SW
(2008) Grape berry plasma membrane proteome analysis and its differential expression during ripening. Journal of Experimental Botany 59, 2979–2990.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zharkikh A,
Troggio M,
Pruss D,
Cestaro A, Eldrdge G ,
et al
.
(2008) Sequencing and assembly of highly heterozygous genome of Vitis vinifera L. cv. Pinot Noir: problems and solutions. Journal of Biotechnology 136, 38–43.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zufferey V,
Murisier F, Schultz HR
(2000) A model analysis of the photosynthetic response of Vitis vinifera L. cvs Riesling and Chasselas leaves in the field: I. Interactions of age, light and temperature. Vitis 39, 19–26.




