Combined effects of contrast between poor and rich patches and overall nitrate concentration on Arabidopsis thaliana root system structure
Manuel Blouin A B and Ruben Puga-Freitas AA Equipe Ibios, UMR Bioemco, Université Paris-Est, 61 Avenue du Général De Gaulle, 94010 Créteil, France.
B Corresponding author. Email: blouin@u-pec.fr
Functional Plant Biology 38(5) 364-371 https://doi.org/10.1071/FP10232
Submitted: 29 November 2010 Accepted: 4 March 2011 Published: 2 May 2011
Abstract
The law of correlative inhibition states that roots in a richer environment develop more intensively if other roots of the same plant are in a poorer environment. This probably occurs only when the cost of emitting these roots in the rich patch is compensated by the advantage of having more roots, i.e. in situations where the difference in concentration between rich and poor patches is strong or the overall nutrient amount in the environment is low. For the first time, we tested root system response to combined gradients of contrast between poor and rich patches and of overall NO3– concentration in agar gels. We set up a factorial in vitro experiment crossing contrast (null, weak, strong heterogeneity) with overall NO3– concentration (deficient, optimal, excessive). We observed an increase in ramification density with increasing heterogeneity in deficient situations; but a decrease with increasing heterogeneity in excessive situations. The interaction between overall NO3– concentration and heterogeneity had a significant effect on root ramification density and the distribution of root length in diameter classes. The overall nutrient status of the soil has to be considered to understand the effect of heterogeneity on plant development at the morphological as well as at the molecular level.
Additional keywords: correlative inhibition law, heterogeneity, NO3–, root development and morphology, root length and ramification.
Introduction
Plant species possess several strategies to increase nutrient capture in heterogeneous soils, including root emission into nutrient patches (Robinson 1994b; Hodge 2006). The study of lateral root (LR) emission in environments where NO3– is distributed heterogeneously teaches us that (i) it exists a local stimulatory control, recently proved to be due to the ion NO3– itself (Zhang et al. 1999; Remans et al. 2006), (ii) an increase in LR emission in the rich patches is often associated with an inhibition of LR proliferation in the poor patches (Gersani and Sachs 1992; Robinson 1994b) and (iii) high overall NO3– concentration in the environment inhibits LR response (e.g. Zhang et al. 1999). In the current experiment, we tested the fact that root system response to heterogeneity is dependent on overall NO3– concentration in the soil environment.
Lateral root proliferation has been observed in nutrient rich patches for a variety of soil nutrients, including NO3–, but also NH4+, and PO42– (Drew 1975; Robinson and Rorison 1983; Robinson 1994b). Localised NO3– treatments can stimulate both initiation and elongation of lateral roots (Hackett 1972; Drew and Goss 1973; Granato and Raper 1989). The study by Gersani and Sachs (1992) brought new insights concerning LR emission: the local development of LR is not dependent on the absolute nutrient concentration in the patch, but rather on the relative concentration of nutrient in the rich patch with regard to the poorer one. They proposed the ‘law of correlative inhibition’, namely, that roots in a given environment develop more intensively if the other roots of the same plant are in a poorer rather than in richer environment.
In his Tansley Review on the responses of plants to non-uniform supplies of nutrient, Robinson (1994b) tested this correlative inhibition law on a dataset of more than 100 results. He found that the correlative inhibition law was explaining less than half of the results, probably due to the fact that factors other than nutrient distribution controlled LR emission (Robinson 1994b).
Kotliar and Wiens (1990) have proposed an additional principle to the law of correlative inhibition for explaining root system response to patchy environments. It states that the difference between nutrient concentration in poor (or background soil) and rich patches, also called the degree of contrast (Kotliar and Weins 1990; Lamb et al. 2004) or degree of heterogeneity in our study, should be strong enough to lead to a benefit that overcomes the cost of emitting lateral roots in the patch (Kotliar and Weins 1990). However, an experiment dedicated to test this hypothesis showed that plants did not respond to the differences in contrast between nutrient in the rich patch and the surrounding background (Lamb et al. 2004). These authors concluded that ‘there is some threshold value necessary to trigger an increased response (possibly a combination of factors such as patch value and overall nutrient status) that was not reached’. This was supported by the fact that high NO3– amounts inhibit the LR response (e.g. Zhang et al. 1999), suggesting the existence of a systemic inhibitory control of LR development due to plant internal N status, itself positively correlated with NO3– availability in the environment. However, since high nitrate concentration can have a local stimulatory effect in heterogeneous environment (e.g. Zhang et al. 1999), it is surprising that the stimulation of growth throughout the root system, unrelated to any locally available nutrients, has received so few comments (Robinson 1994b). Because of the view that plants rarely encounter soil nutrient concentrations leading to toxicity, only low nutrient availability conditions have been studied. Considering a wide range of overall nutrient concentrations is necessary to develop a general theory for plant response to variations in resource availability in the environment. A synthetic conceptual model has been proposed by de Kroon et al. (2009): plants foraging for resources in heterogeneous environments must involve (1) plasticity at the level of individual modules in reaction to localised environmental signals and the potential for modification of these responses either, (2) signals received from connected modules that may be exposed to different conditions (law of correlative inhibition), or (3) signals reflecting the overall resource status of the plant, explaining the negative feedbacks in excess nutrient conditions. Straightforward predictions from this conceptual model are that a local response to resource abundance will be enhanced when many other integrated modules are growing under conditions of resource shortage, and that the whole plant experiences a nutrient shortage, and that this will result in elevated resource uptake at a local scale (de Kroon et al. 2009). Conversely, the local response will be reduced when foraging efforts are unlikely to improve an already favourable plant resource status (Lamb et al. 2004). We propose as an additional prediction that LR emission could even decrease when NO3– is in excess and becomes toxic, as a way to reduce root system surface area and the exposure of cells to the toxic ion.
In an in vitro experiment with a 3 × 3 factorial design, we study LR emission of Arabidopsis thaliana (L.) Heynh. along a gradient of NO3– distribution heterogeneity (null, weak or strong heterogeneity) crossed with an overall gradient of NO3– amounts in the environment (deficit, optimal or excess) to determine if LR density and root length were affected by (i) the level of heterogeneity of NO3– distribution, (ii) the overall N amount in the soil, and (iii) the interaction between heterogeneity and the overall N amount in the soil.
Materials and methods
Microcosms
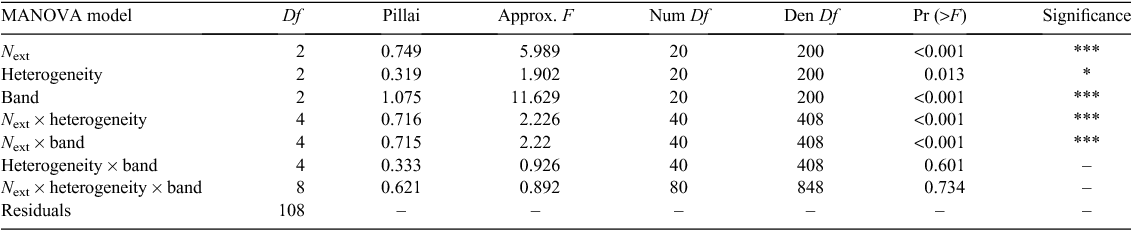
Our experimental design was set up to study the effect of the global N concentration in the environment and the heterogeneity of its distribution. We applied localised NO3– treatments to Arabidopsis thaliana (L.) Heynh. with the segmented agar plate set-up accordingly to Zhang and Forde (1998), except that we used square transparent boxes instead of circular Petri dishes, which permitted the use of equal gel volumes in the top, middle and lower patches and made the vertical positioning easier. Gels were made of a 9 g L–1 gelrite, which represents a relatively high gel strength, similar to that which plants can experiment in soils, with a classical Murashige and Skoog medium (Murashige and Skoog 1962) with micronutrients and vitamin at standard concentrations. Macronutrient concentrations were kept standard for CaCl2 = 2.99 mM, KH2PO4 = 1.25 mM and MgSO4 = 1.50 mM. NO3– was brought at a basal level as NH4NO3 = 10 µM (instead of 20.61 mM in MS medium). The KNO3 concentration was adapted according to the treatment, and the K concentration was kept constant by adding KCl, which has no effect on root system structure (Zhang et al. 1999). Gels with different KNO3 concentrations were prepared, cut in bands (width: 10 cm, height: 2 cm, thickness: 1 cm, i.e. 20 mL per band) and placed in new boxes with a small plastic grid with a 1 mm mesh size between two bands to avoid direct contact and KNO3 diffusion. The overall NO3– concentration (or amount, since volume was constant) in the whole culture medium (called Next hereafter) presented three levels (Fig. 1): (A) the deficit treatment with 0.5 mM of NO3– in 60 mL, i.e. 3.10−5 moles, (B) the optimal treatment with 5 mM in 60 mL, i.e. 3.10−4 moles and (C) the excess treatment with 50 mM in 60 mL, i.e. 3.10−3 moles. Heterogeneity corresponded to the difference in concentration/amount of NO3– between the poor bands (a and c) and the rich band (b). This factor presented also three levels (Fig. 1): (0) the null heterogeneity corresponded to a situation where the a, b and c bands had a identical NO3– amount equal to 2/6 of the overall NO3– amount, (1) the weak heterogeneity to a situation where the a and c bands had a NO3– amount equal to 1/6 and the b band equal to 4/6, and (2) the strong heterogeneity to a situation where the a and c bands had a NO3– amount equal to 0/6 and the b band equal to 6/6. There were nine different treatments, each replicated five times.
|
Plant culture
Seeds of A. thaliana cv. Columbia were sterilised for 10 min in Teepol HB7 (Sigma–Aldrich Chemie, Steinheim, Germany) and 10 min in 90% ethanol. They were sown in MS medium at 0.5 mM, the NO3– concentration used in the deficit homogeneous treatment A0. After 15 days, 45 similar seedlings with a seminal root 2.3–2.5 cm long were transferred in the experimental set-up by positioning the root between the gel and the box. The end of the root (0.3–0.5 cm) was placed behind the b band, whereas the major part (2 cm) was behind a band. The pretreatment was essential to obtain seedlings with a main root of 2.3–2.5 cm, to put the root in contact with the second band. If seeds were directly set in the experimental device it is probable that some would not have developed enough to reach the second band, leading to the absence of heterogeneity effects (Zhang and Forde 1998). After the transfer, the concentrations experienced by plants were different in the three heterogeneity treatments at a given Next concentration. This was the only way to keep the same overall N concentration independent of the heterogeneity. Plants were grown for 26 days in an in vitro culture chamber at 18°C, with a 12 h photoperiod (light intensity: 200 µmol photons s–1).
Root system analysis
Each root system was analysed with a digital scanner (Epson Expression 10000 XL, Epson America Inc., San Jose, CA, USA) coupled with the WinRHIZO software (WinRHIZO, V. 2007 pro, Regent Instruments, Quebec, Canada). As advised by sensitivity analyses of this material and software (Bouma et al. 2000; Himmelbauer et al. 2004), we used a double light system, at a resolution of 16 p mm–1 (400 dpi), with the automatic transformation threshold option which optimises the distinction of pixels considered as root or background according to their relative contrast. Eleven equal diameter classes were defined: from 0–100 to 800–900 and >900 µm. After root system analysis, both above- and belowground organs were dried for 2 days at 45°C and weighed.
Numerous variables characterising root systems can be obtained with image analysis. We were specifically interested with the emission of lateral roots. The number of lateral roots in each band can be considered a good indication of the emission of lateral roots, but because the number of ramifications is correlated with root system size, we measured the root biomass for each band to obtain the ramification number per unit biomass, hereafter called ‘ramification density’. This variable was more relevant to our research question than the ramification number per unit length; indeed, we investigated a wide range of N concentrations which could have an impact on the tissue density. This change in tissue density was not taken into account in the ramification number per unit length, but in the ramification number per unit biomass. We were also interested in the length of lateral roots, which could respond differently than the emission of lateral roots to the different experimental factors. So we analysed the length per diameter classes.
Results
Plant biomasses
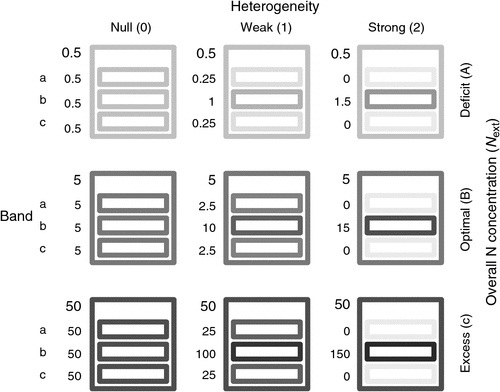
Belowground, aboveground and total dry biomasses were significantly affected by the overall N concentration in the culture medium (Next) but not by heterogeneity or by the interaction between Next and heterogeneity (Table 1). Shoot : root ratio was affected by Next, and less significantly by heterogeneity (Table 1). Total, below and aboveground biomasses were slightly increased at the ‘optimal’ Next compared with the ‘deficit’ Next, but the difference was not significant (Fig. 2). However, root biomass decreased significantly in ‘excess’ Next compared with ‘optimal’ and ‘deficit’ Next. A significant increase in the shoot : root ratio was detected from the deficit to the excess Next treatment.
|
Root system structure
Root system parameters were analysed band per band (c.f. ‘Materials and methods’), because the local N concentration was not the same in each band (the rich band was always the middle band called ‘b’, whereas the top band ‘a’ and down band ‘c’ were equally poor). A three-way ANOVA with the factors ‘overall N concentration in the environment’ (Next), ‘heterogeneity’ and ‘band’ was realised to detect a potential effect of Next, heterogeneity or their interaction on root system structure, with band as a cofactor explaining variations due to the age of the roots. First, the ‘band’ factor had a significant effect on all parameters describing root system structure (length, ramification number, ramification density); moreover, the interaction between band and Next was also significant for these parameters. This result justified the addition of this factor to the analysis, to be able to detect the effect of other factors. The number of ramifications was significantly affected by the factor Next (P = 2.13 × 10−12) but not by heterogeneity (P = 0.69), or by the interaction between Next × heterogeneity (P = 0.41). The ramification number per unit biomass (ramification density) integrated the fact that root system size and/or tissue density could differ between treatments (see ‘Materials and methods’ for more details). Ramification density (Table 2) was significantly affected by Next (P = 9.38 × 10−5), and also by heterogeneity (P = 0.028) and the interaction heterogeneity × Next (P = 0.015). This was more significant for the second and superior ramification orders than for the first one (data not shown). We reported ramification density according to the different treatments of overall N concentration and heterogeneity levels in Fig. 3. In this figure, it can be noticed that the highest level of ramifications per unit biomass was reached in the treatment A1c (deficit of N, weak level of heterogeneity, bottom band) and the lowest in the C2a and C2c (excess of N, strong heterogeneity, bands a and c respectively). A general trend was an increase in ramification density with the position of the band (from a to c), probably due to the fact that young root in the c band was finer and more responsive to environmental heterogeneity than older roots from bands a and b; this is typical from a developmental effect (Robinson 1994b). The variation of ramification density between the three bands of one experimental unit was small in the null heterogeneity treatment (heterogeneity = 0), but strong in the weak and strong heterogeneity treatments (heterogeneity = 1 and 2) (Fig. 3). Concerning the direction of these variations, all treatments with a deficit in overall N concentration (Next = A) exhibited an increase in ramification density between the bands a and b and between the bands b and c. The treatments with an optimal overall N concentration (Next = B) exhibited the same pattern, except the null heterogeneity treatment (B0) where the NO3– amount in the band a was already high (see Fig. 1). The treatments with an excess in overall N concentration (Next = C) exhibited a pattern of variation dependant on the level of heterogeneity: a constant ramification density at null heterogeneity (C0), an increase from a to c at weak heterogeneity (C1) and a decrease from bands b to c at high heterogeneity (C2). The interaction between Next and heterogeneity was clear when comparing the three overall N concentrations (Next = A, B and C) at strong heterogeneity (heterogeneity = 2). It can, thus, be observed that heterogeneity can induce opposite effects on ramification density according to the overall amount of N in the environment.
|
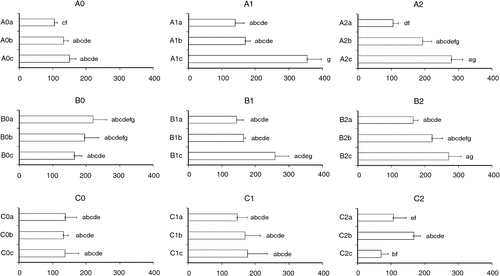
|
Root system structure was also analysed in term of length percentage per diameter class, with diameter classes with a range of 100 µm, from 0 to 900 µm. By analysing the result for each one of the nine classes in a three-way ANOVA with Next and heterogeneity as factors and band as cofactor, we observed that Next affected significantly all diameter classes (with a P < 0.0001), that heterogeneity affected significantly the classes 0–100 (P < 0.05), 100–200 (P < 0.10), 400–500 (P < 0.001) and 500–600 µm (P < 0.10), and that the interaction between Next and heterogeneity affected significantly the classes 0–100 (P < 0.0001), 100–200 (P < 0.05), 200–300 (P < 0.10), 300–400 (P < 0.05), 500–600 µm (P < 0.10) and 600–700 µm (P < 0.10). It can be noted that the interaction between Next and heterogeneity was significantly affecting root diameter classes more than the heterogeneity by itself. These lengths per diameter classes are reported in Fig. 4. The first graph with the absolute length shows that treatments B1 and B2 were those with the longest roots in all classes, whereas treatments with excess overall N amount (C) were those with the smallest length. On the second graph, these data were reported on the total root system length to analyse qualitative changes. All treatments with an excess Next (C0, C1 and C2) and the treatment B0 exhibited a development divergent from the other treatments: the length percentage of fine roots (from 0 to 300 µm) was smaller, whereas the length percentage of coarse roots (from 400 to 700 µm) was higher than in other treatments. The distribution of length among all diameter classes taken together was analysed in a MANOVA (Table 3). Significant effects were observed for Next and heterogeneity as well as for the Next × heterogeneity interaction, confirming that the effect of heterogeneity was dependant on the overall N amount in the environment. Here again, it can be noted that the effect of the Next × heterogeneity interaction was stronger than that of heterogeneity, as shown by the value of the Pillai-M. S. Bartlett trace test statistics (0.72 and 0.32, respectively) and the P-values (0.00005 and 0.01, respectively).
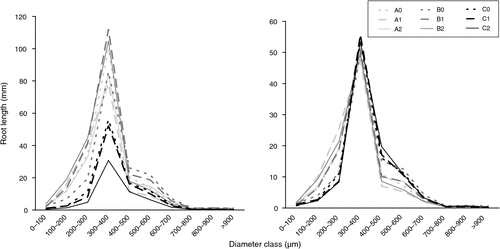
|
Discussion
This study was dedicated to investigate root system structure along a gradient of overall NO3– concentrations crossed with a gradient of NO3– heterogeneity distribution. The dicotyledon species A. thaliana has a root system developing from one main root, the radicle. A split root system with two equivalent root system parts grown in vertical agar bands was, thus, not appropriate. Consequently, agar bands were oriented horizontally (Zhang and Forde 1998) and plant roots were reaching the different horizontal bands at different ages and development stages. As a result, one major effect observed in our experiment was a ‘band’ effect (Tables 2, 3), strongly correlated with root age. As in several previous studies, we observed a lateral root response not strictly localised in the rich patch. This ‘time-dependant responses’ have been reviewed by Robinson (1994b); for example, when NO3– is applied to a discrete location in soil with wheat plants that had already begun ear development, roots did proliferate, but throughout the root system, not just around the point of NO3– application (Robinson 1994a). Younger plants did respond as expected (Robinson 1991). This effect of age made the results more complex (Figs 2, 3), but it can be considered as a covariable in ANOVA to estimate the significance of factors such as Next, heterogeneity and Next × heterogeneity which is discussed below.
Overall N concentration significantly affected root system structure in our experiment (Tables 1–3). It was difficult to observe a general trend in a 3× 3 factorial design; this was probably due to the age effect discussed above and to the pretreatment at 0.5 mM for the germination of all seeds, before their removal in the experimental units with different nitrogen concentrations. However, it can be perceived that the number of ramifications per root biomass unit was constant from deficient to optimal Next, but it decreased from optimal to excessive Next (Fig. 3). Root length increased from deficient to optimal Next and then decreased from optimal to excessive Next (Fig. 4). This is in accordance with the fact that in high nutrient concentration situations the general trend is a decrease in root length and number of ramification at excessive nutrient supply. The most common response to an increase in nitrate and ammonium concentration in the environment is a decrease in root : shoot ratio (Boxman et al. 1991; Wang and Below 1996; Bauer and Berntson 1999) and a decrease in fine : coarse root ratio (Haynes and Goh 1978; Boxman et al. 1991; Britto and Kronzucker 2006), as observed here.
The degree of heterogeneity in nutrient distribution, also called the degree of contrast or the difference in nutrient availability between the patch and the background soil (Kotliar and Weins 1990; Lamb et al. 2004), has received little research attention. Models examining nutrient uptake from heterogeneous soils predict an increase in the root proliferation and in the biomass of adapting plants when contrast increases (Jackson and Taylor 1996; Fransen et al. 1999). To our knowledge, experimental testing of this theory is limited to the studies by Wijesinghe and Hutchings (1999) and Lamb et al. (2004). In an experiment with the plant Abutilon theophrasti, neither root and shoot biomasses nor root proliferation was affected by patch/background contrast (Lamb et al. 2004). Authors interpreted this as a potential consequence of ‘some threshold value necessary to trigger an increased response (possibly a combination of factors such as patch value and overall nutrient status) that was not reached’. They concluded that ‘Validation of this model will require similar experiments using a much wider range of contrasts and total nutrient availability’ (Lamb et al. 2004). In the other experiment, the clonal herb Glechoma hederacea, a species that benefits strongly from heterogeneous soil (Birch and Hutchings 1994), increasingly concentrates root biomass in rich patches as the contrast increases, while total shoot biomass was similar between contrast treatments (Wijesinghe and Hutchings 1999). In the same way, in our experiment, we observed a significant effect of heterogeneity on ramification density and root length per diameter classes (Tables 2, 3). This effect of heterogeneity was associated with an increase in total biomass only in the optimal overall N concentration (Fig. 1). It is likely that in the study by Lamb et al. (2004), the contrast was not strong enough, or that the effect of overall N concentration was compensating the effect of contrast. In the study by Wijesinghe and Hutchings (1999), the contrast was strong enough to observe changes in root proliferation, but the overall N concentration was probably not appropriate to observe a change in plant biomass.
Finally, the most important result of our study was that root system response to heterogeneity depended on the overall NO3– concentration in the environment, as shown by the significant effect of the Next × heterogeneity interaction on ramification density and root length (Tables 2, 3; Figs 3, 4). The effect of the Next × heterogeneity interaction was even more significant than the effect of heterogeneity itself (Tables 2, 3). Heterogeneity had a positive effect on ramification density when the overall NO3– amount in the environment was limiting or optimal, but a negative effect when NO3– was in excess. This can be interpreted as a way to maximise the root surface area when a nutrient is limiting, and to minimise it when it becomes toxic.
The significant interaction between overall NO3– concentration in the environment and the degree of heterogeneity could explain why so few studies reviewed by Robinson (1994b) seem to follow the law of correlative inhibition. We suggest that if authors systematically mentioned the overall nutrient amount in the environment in addition to the contrast between poor and rich patches, it would be easier to overcome the apparent contradiction between experiments.
Acknowledgements
We thank Danielle Benest for technical and the ‘Ingénierie Ecologique’ program of the CNRS ‘Institut Ecologie et Environnement’ for funding this study.
References
Bauer GA, Berntson GM (1999) Ammonium and nitrate acquisition by plants in response to elevated CO2 concentration: the roles of root physiology and architecture. Tree Physiology 21, 137–144.Birch CPD, Hutchings MJ (1994) Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea. Journal of Ecology 82, 653–664.
| Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea.Crossref | GoogleScholarGoogle Scholar |
Bouma TJ, Nielsen KL, Koutstaal B (2000) Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant and Soil 218, 185–196.
| Sample preparation and scanning protocol for computerised analysis of root length and diameter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhvVGrtb8%3D&md5=81261ae115b4dbab1472211f511c934aCAS |
Boxman AW, Krabbendam H, Bellemakers MJS, Roelofs JGM (1991) Effects of ammonium and aluminium on the development and nutrition of Pinus nigra in hydroculture. Environmental Pollution 73, 119–136.
| Effects of ammonium and aluminium on the development and nutrition of Pinus nigra in hydroculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXlsFOqu74%3D&md5=8a5a8766cb878ba3f071cbbf7f230fd8CAS | 15092085PubMed |
Britto DT, Kronzucker HJ (2006) Futile cycling at the plasma membrane: a hallmark of low-affinity nutrient transport. Trends in Plant Science 11, 529–534.
| Futile cycling at the plasma membrane: a hallmark of low-affinity nutrient transport.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFeru7%2FL&md5=9c59f9f6b334adf5d522dccf6477b01cCAS | 17035071PubMed |
De Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ (2009) A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant, Cell & Environment 32, 704–712.
| A modular concept of plant foraging behaviour: the interplay between local responses and systemic control.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmslemsLk%3D&md5=a738255ad5e2099ebf7cb2fa5b2fa970CAS | 19183298PubMed |
Drew MC (1975) Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist 75, 479–490.
| Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XlslOkug%3D%3D&md5=8df94a7f34b85ec239c603c0973ab8bfCAS |
Drew MC, Goss M (1973) Effect of soil physical factors on root growth. Chemistry & Industry 14, 679–684.
Fransen B, De Kroon H, De Kovel CGF, Van den Bosch F (1999) Disentangling the effects of root foraging and inherent growth rate on plant biomass accumulation in heterogeneous environments: a modelling study. Annals of Botany 84, 305–311.
| Disentangling the effects of root foraging and inherent growth rate on plant biomass accumulation in heterogeneous environments: a modelling study.Crossref | GoogleScholarGoogle Scholar |
Gersani M, Sachs T (1992) Developmental correlations between roots in heterogeneous environments. Plant, Cell & Environment 15, 463–469.
| Developmental correlations between roots in heterogeneous environments.Crossref | GoogleScholarGoogle Scholar |
Granato TC, Raper CDJ (1989) Proliferation of maize (Zea mays L.) roots in response to localized supply of nitrate. Journal of Experimental Botany 40, 263–275.
| Proliferation of maize (Zea mays L.) roots in response to localized supply of nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXktVCgtb0%3D&md5=54a9ae4d1f5e88f8ed4fc9b803e55f61CAS | 11542157PubMed |
Hackett C (1972) A method of applying nutrients locally to roots under controlled conditions and some morphological effects of locally applied nitrate on the branching of wheat roots. Australian Journal of Biological Sciences 25, 1169–1180.
Haynes RJ, Goh KM (1978) Ammonium and nitrate nutrition of plants. Biological Reviews of the Cambridge Philosophical Society 53, 465–510.
| Ammonium and nitrate nutrition of plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXosFOhsQ%3D%3D&md5=359a036267d0aca7cc5a1eb39b210861CAS |
Himmelbauer ML, Loiskandl W, Kastanek F (2004) Estimating length, average diameter and surface area of roots using two different image analyses systems. Plant and Soil 260, 111–120.
| Estimating length, average diameter and surface area of roots using two different image analyses systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksFGltLg%3D&md5=171acceb398b743b8c0c4d833d7d7509CAS |
Hodge A (2006) Plastic plants and patchy soils. Journal of Experimental Botany 57, 401–411.
| Plastic plants and patchy soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XisFKgsw%3D%3D&md5=7bedfd2e15e5343404426123c67f263bCAS | 16172138PubMed |
Jackson AO, Taylor CB (1996) Plantmicrobe interactions: life and death at the interface. The Plant Cell 8, 1651–1668.
Kotliar NB, Weins JA (1990) Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos 59, 253–260.
| Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity.Crossref | GoogleScholarGoogle Scholar |
Lamb EG, Haag JJ, Cahill JFJ (2004) Patch-background contrast and patch density have limited effects on root proliferation and plant performance in Abutilon theophrasti. Functional Ecology 18, 836–843.
| Patch-background contrast and patch density have limited effects on root proliferation and plant performance in Abutilon theophrasti.Crossref | GoogleScholarGoogle Scholar |
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497.
| A revised medium for rapid growth and bio-assays with tobacco tissue cultures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3sXksFKm&md5=06c472aaff717f5157aa29da67ee5ad0CAS |
Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, Tillard P, Forde BG, Gojon A (2006) The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proceedings of the National Academy of Sciences of the United States of America 103, 19206–19211.
| The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlChsrjO&md5=da4b683f3639b2669bccf24c20c8461dCAS | 17148611PubMed |
Robinson D (1991) The efficiency of crop root-systems in nutrient uptake. In ‘Scottish Crop Research Institute Annual Report 1991’. pp. 49–51. (Scottish Crop Research Institute: Dundee, Scotland)
Robinson D (1994a) Resource capture by single roots. In ‘Resource capture by crops’. (Eds JL Monteith, RK Scott, MH Unsworth) pp. 53–76. (Nottingham University Press: Nottingham, UK)
Robinson D (1994b) The responses of plants to non-uniform supplies of nutrients. New Phytologist 127, 635–674.
| The responses of plants to non-uniform supplies of nutrients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmsFCmu7Y%3D&md5=3c3be12a117f09ece8b325c053af6da5CAS |
Robinson D, Rorison IH (1983) A comparison of the responses of Lolium perenne L., Holcus lanatus L. and Deschampsia flexuosa (L.) Trin. to a localised supply of nitrogen. New Phytologist 94, 263–273.
| A comparison of the responses of Lolium perenne L., Holcus lanatus L. and Deschampsia flexuosa (L.) Trin. to a localised supply of nitrogen.Crossref | GoogleScholarGoogle Scholar |
Wang XT, Below FE (1996) Cytokinins in enhanced growth and tillering of wheat induced by mixed nitrogen source. Crop Science 36, 121–126.
| Cytokinins in enhanced growth and tillering of wheat induced by mixed nitrogen source.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XotVyjtw%3D%3D&md5=c3ceee398d1717ea2db0230da07ce048CAS |
Wijesinghe DK, Hutchings MJ (1999) The effects of environmental heterogeneity on the performance of Glechoma hederacea: the interactions between patch contrast and patch scale. Journal of Ecology 87, 860–872.
| The effects of environmental heterogeneity on the performance of Glechoma hederacea: the interactions between patch contrast and patch scale.Crossref | GoogleScholarGoogle Scholar |
Zhang H, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279, 407–409.
| An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmsVShtA%3D%3D&md5=5864fb349ffd34fbbede0d80791da0daCAS | 9430595PubMed |
Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proceedings of the National Academy of Sciences of the United States of America 96, 6529–6534.
| Dual pathways for regulation of root branching by nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXksFKktbc%3D&md5=9030529225d6c376f42adfe4602b5c77CAS | 10339622PubMed |