Root responses to neighbouring plants in common bean are mediated by nutrient concentration rather than self/non-self recognition
Eric A. Nord A , Chaochun Zhang B and Jonathan P. Lynch A C DA Department of Horticulture, The Pennsylvania State University, University Park, PA 16802, USA.
B Department of Plant Nutrition, China Agricultural University, Beijing, 100193, PR China.
C Intercollege Program in Ecology, The Pennsylvania State University, University Park, PA 16802, USA.
D Corresponding author. Email: JPL4@psu.edu
Functional Plant Biology 38(12) 941-952 https://doi.org/10.1071/FP11130
Submitted: 26 May 2011 Accepted: 12 August 2011 Published: 19 October 2011
Abstract
Plants are reported to over-proliferate roots in response to belowground competition, thereby reducing reproductive biomass. This has been cited as an instance of the ‘tragedy of the commons’. Many of the studies that report this response suggest that plants can sense neighbours and discriminate between ‘self’ and ‘non-self’ roots. To test the alternate hypothesis that root responses to a neighbouring plant are mediated by resource depletion, common bean plants were supplied with the same phosphorus (P) fertiliser dose in varying rooting volumes, or with neighbouring plants separated by plastic film, nylon mesh, or no barrier to vary access to a neighbour. Phosphorus concentration, but not the presence of a neighbour or rooting volume, strongly influenced biomass allocation to roots. Root architecture was significantly altered by both neighbours and P availability. When exposed to the roots of a neighbour, plants altered the vertical and horizontal distribution of roots, placing fewer roots in soil domains occupied by roots of a neighbour. These results support the hypothesis that root responses to neighbouring plants are mediated by resource depletion by the neighbour rather than sensing of ‘non-self’ roots and show that the presence of a neighbour may affect root architecture without affecting biomass allocation to roots.
Additional keywords: nutrient uptake, P, root, root architecture, self/non-self discrimination.
Introduction
Competition – both above- and belowground – is a major factor shaping plant communities and is thought to be partly responsible for the diversity of vegetation in different ecosystems (Goldberg and Barton 1992; Wilson and Tilman 2002; Moore 2003; Passarge et al. 2006). The intensity of above- and belowground competition depends on the availability of nutrients and light in the environment (Wilson and Tilman 1993) and above- and belowground responses to competition may be independent (Murphy and Dudley 2007). However, compared with aboveground competition, root competition is less well understood since it is affected by multiple soil resources (Casper and Jackson 1997) as well as by animals (Endlweber and Scheu 2006) and is difficult to observe directly.
Interactions may occur among roots of an individual plant (intraplant or self competition) or between roots of different plants (interplant or non-self competition). Root architecture may have important effects on the intensity of both intra- and interplant competition. Steeper basal root angles in common bean resulted in more intensive intraplant competition (Ge et al. 2000; Rubio et al. 2001) and more similar root architecture leads to more intensive interplant competition according to geometric modelling (Rubio et al. 2001, 2003). Interplant root competition leads to over-production of roots and consequently to a reduction in reproductive biomass; this root over-proliferation response (ROR) has been cited by others as an instance of ‘the tragedy of the commons’ (Gersani et al. 2001). The over-proliferation of roots in the presence of a neighbour is reported to be evidence of the ability of plants to differentiate between their own roots (self) and the roots of a competitor (non-self) (Gersani et al. 2001; Falik et al. 2003). Schenk (2006) demonstrated that the self/non-self studies of Gersani et al. (2001) could be simply explained by taking soil volume into account. Hess and de Kroon (2007) reanalysed data from several published studies and concluded that plant responses to the available soil volume could explain some of the results that had been attributed to competition. In several experiments that were not confounded by volume there was an increase in root mass that depended solely on the identity of neighbours (de Kroon et al. 2003; Holzapfel and Alpert 2003; Gruntman and Novoplansky 2004). However, these non-confounded studies do not show a subsequent decrease in shoot biomass or seed production, so the importance of ROR in belowground competition has mixed experimental support. Indeed, it is not clear whether competition has occurred in these non-confounded experiments, since the more restrictive definitions of competition require that the ‘growth, survival, or fecundity’ of neighbours be reduced (Casper and Jackson 1997). Neither is it fully clear by what mechanism the presence of a neighbour affects the growth of individual plants.
Competition for nutrients may play an important role in root interactions, but its effect is often confounded by differences in nutrient availability and effective rooting volume (McConnaughay and Bazzaz 1991; Matthes-Sears and Larson 1999). Plant responses to soil volume could confound the interpretation of the effects of competition (Schenk 2006; Semchenko et al. 2007). O’Brien and Brown (2008), in a mathematical model, consider the separate effects of volume per plant (V) and nutrient concentration per unit volume (N) on root proliferation and the outcome of competition and conclude that when N is held constant, increasing V should produce an almost linear increase in root proliferation and net nutrients available for reproduction; When V × N is held constant, roots per plant should at first increase and then decline with increasing volume. Nutrient concentration and soil volume used in experiments should, therefore, be explicitly considered in order to clarify the outcome of competition. We hypothesised that nutrient availability rather than the presence of a neighbour itself affects biomass allocation.
Phosphorus is nearly immobile in soil (Barber 1995) and its absorption by roots results in localised depletion zones, thereby influencing local P availability at a millimetre scale (Lynch 1995). The P depletion volume is positively correlated with root length, as well as other root traits, such as basal root gravitropism (Ge et al. 2000; Lynch and Brown 2001; Rubio et al. 2001; Walk et al. 2004). Resource availability at this scale should affect belowground resource competition. It is possible that many of the responses to the presence of a neighbour attributed to plants sensing the presence of a neighbour’s roots may actually be mediated by root responses to local nutrient availability, especially the immobile nutrients such as P, and plants may not be able to distinguish between self competition and non-self competition. Phosphorus availability rather than competition may determine root biomass allocation.
Most studies addressing the effects of belowground competition focus on root biomass but overlook root architecture. Because P mobility in soil is diffusion-limited and because P is generally concentrated in the epipedon, P acquisition is very sensitive to the location of root foraging. For this reason root architecture may play a more important role in P acquisition than root biomass. Several factors affect P acquisition by plants, including rhizosphere modification, root morphology and root architecture (Hinsinger et al. 2005; Lynch and Brown 2006). There is much physiological evidence that root architecture responds to P availability (Bonser et al. 1996; Williamson et al. 2001; Linkohr et al. 2002; Chevalier et al. 2003; Miller et al. 2003; Jiang et al. 2007). Various root architectural traits increase P acquisition by enhancing topsoil foraging (Lynch and Brown 2001) and patch exploitation (Borch et al. 1999; Farley and Fitter 1999); these include basal root growth angle (Bonser et al. 1996; Liao et al. 2001; Ho et al. 2005), production of shoot-borne roots (Miller et al. 2003; Walk et al. 2006), increased axial elongation (Ma et al. 2003) and reduced lateral branching in low P domains (Borch et al. 1999). In contrast, competition for P may elicit changes in root architecture, thereby facilitating resource partitioning (Callaway et al. 2003). Lynch (2005) points out that the changes in root architecture induced by low P availability can be interpreted as precision foraging. Such precision foraging by roots could lead to root avoidance as roots preferentially avoid regions where soil nutrients have been depleted by other roots. This would tend to reduce resource competition among neighbouring roots. Since root architectural changes do not necessarily imply altered allocation of photosynthate to the root system, altering root architecture should be ‘cheaper’ than increasing biomass allocation to roots and root architecture may be expected to exhibit greater plasticity in response to the presence of a neighbour than will root biomass. It is hypothesised that root architecture should respond to the presence of a neighbour much earlier than root biomass.
In summary, we propose two hypotheses: (1) soil P concentration rather than the presence of a neighbour will determine biomass allocation to roots; and (2) root architecture will exhibit greater plasticity to the presence of a neighbour than will root biomass. In order to test these hypotheses, we conducted two experiments with common bean (Phaseolus vulgaris L.), one to test the importance of nutrient concentration and the presence of a neighbour and another to characterise root architectural responses to the presence of a neighbour.
Materials and methods
Experiment 1: Effects of P dose, P concentration and neighbour
Experimental design
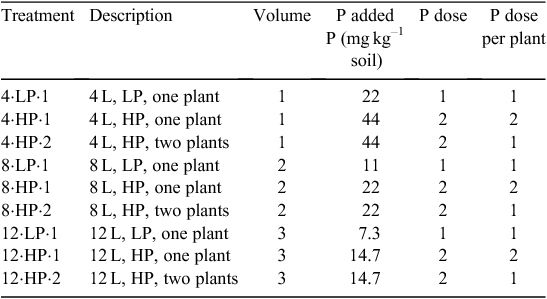
In this experiment, two P dosages (low P = 84 mg pot–1 and high P = 164 mg pot–1 of P) were added to each of three soil volumes (4, 8 and 12 L), resulting in five concentrations of P (7.3, 11, 14.7, 22 and 44 mg P kg–1 soil, see Table 1). There were two neighbour treatments (one plant or two plants per pot); pots with one plant (no neighbour) received both the low P and high P dosages, whereas pots with two plants (with neighbour) received the high P dosage. In this way, each of two plants with high P would have the same P available per plant as the single plant in low P (Table 1). Each treatment was replicated four times.
|
Plant growth
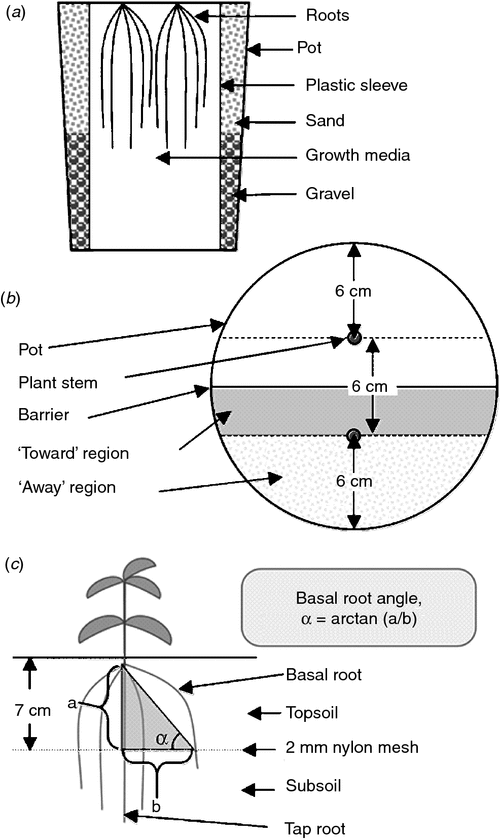
The growth medium consisted of 50% sand, 35% coarse vermiculite and 15% red soil (C horizon from a limestone-derived silt loam; fine, mixed, semiactive, mesic Typic Hapludalf). The red soil was oven dried and finely powdered before mixing with the other media components. The characteristics of this solid media were: pH 6.9 (1 : 1 = soil : water), Olsen-extracted P 4.0 mg kg–1 soil, CEC 4.5 mEqn 100 g–1. Three sizes of plastic (polyethylene) sleeves were used to achieve volumes of 4, 8 or 12 L. The height of all plastic sleeves was constant so that soil column height was the same for all pot sizes, but the diameter was varied to achieve the desired volume. Each plastic sleeve was placed inside a 12 L pot and then was filled with medium into which either one or two doses of P as finely ground triple super phosphate (0–46–0) had been thoroughly mixed according to the experimental design (Table 1). The space between the pot and plastic sleeve was filled with sand and gravel to support the sleeve and to assure that all pot sizes had equivalent thermal mass (Fig. 1a).
|
Uniform seeds of common bean (Phaseolus vulgaris L., RIL 30 from the L88 population developed by J. Kelly at Michigan State University), were surface sterilised, scarified and germinated in rolls of brown germination paper (Anchor Paper Co., St Paul, MN, USA) saturated with 0.5 mM CaSO4 for 2 days at 25°C before planting. All pots were irrigated daily using an automatic drip system which delivered 100 mL day–1 of a modified nutrient solution (Epstein 1972) that lacked P and consisted of 3.1 mM NO3, 1.8 mM K, 1.2 mM Ca, 1.4 mM SO4, 1.0 mM NH4, 0.825 mM Mg, 0.05 mM Cl, 5 µM Fe-EDTA, 2 µM B, 1.5 µM Mn, 1.5 µM Zn, 0.143 µM Mo and 0.5 µM Cu. Nutrient solution pH was adjusted to 5.5–5.8. Plants were grown between April and June 2005 in a climate controlled greenhouse at University Park, Pennsylvania, USA (40°49′N, 77°49′W). The average temperature was 24°C ranging from a maximum of 28°C (day) to a minimum of 22°C (night), the photoperiod was 14/10 h (day/night) and the maximum midday photosynthetic flux densities reached 1000 µmol photons m–2 s–1.
Biomass, P and root measurements
Plants were harvested 28 days after planting (DAP). A representative sample of each root system was selected by removing the roots from approximately one-quarter of the media. These subsamples were scanned and the resulting images were analysed to calculate root length using image analysis software (WinRhizo Pro 2002, Regent Instruments, Quebec, Canada). The remainder of the roots were washed from the soil, dried and weighed. After scanning, root subsamples were dried at 60°C for 48 h and weighed. Specific root length (root length per gram root biomass) was calculated from the subsample root biomass and root length and total root length calculated from total root system dry mass and specific root length. Shoot and root tissue were dried at 60°C for 2 days and weighed. Tissue P concentration was determined spectrophotometrically (Murphy and Riley 1962). The allometric partitioning coefficient k, was calculated as the slope of the regression of the logarithm of root biomass on the logarithm of shoot biomass (Niklas and Enquist 2002).
Depletion zone fraction was estimated from the depletion zone radius and root length. The radius of the P depletion zone; which typically extends about 1 mm from the root surface (Gahoonia and Nielsen 1992) was estimated from the P diffusion rate and age of the root (Ge et al. 2000), as follows for each possible age of roots:

where rdz is the depletion zone radius (cm); rr is the root radius (cm); De is the effective diffusion coefficient for P (here De = 10−8 cm2 s–1); and t is the age of the root(s). We partitioned the total root length according to root age based on the assumption of exponential growth in root length.
Phosphorus depletion zone volume was then calculated as follows for each age of root:

where L is root length; and V is the total volume of pot used in the experiment. These were summed over all root ages and divided by the total soil volume to determine the P depletion volume fraction.
Statistical analysis
All treatments were replicated four times. Data were analysed in R ver. 2.10 (R development core team 2010), with mixed effects models using the package ‘nlme’ (Pinheiro et al. 2009), with replicate modelled as a random effect. When two plants were grown together, the average biomass and root length were reported for comparison with single plants. All interactions that could be tested were included, but because this experiment was not fully factorial (two plants in low P were not included), not all interactions could be tested. To ensure that potentially important interactions were not overlooked, models were fit by beginning with a full model and removing non-significant effects, following procedures outlined by Crawley (2005) and Zuur et al. (2009). The effect of P dose and the presence of a neighbour were tested by comparing specific pairs of treatments using mixed models, rather than testing all treatments (Table 2).
Experiment 2: Effects of P stress and neighbour
Experimental design
Two plants were grown in each pot with one of three neighbour treatments: (i) no neighbour (‘Isolated’) – pot divided into two root zones with a barrier of polyethylene allowing no root interaction; and (ii) partial access to neighbour (‘Partial’) – pot divided with a 40 µm nylon mesh barrier (Anping Hengxing Bolting Cloth Co. Ltd, Hebei, China) allowing soil solution to move freely but confining roots; and (iii) full access to neighbour (‘Full’) – no barrier, roots free to intermingle.
Since two plants were grown in every pot, all plants experienced similar levels of aboveground competition. Because the mesh used in the ‘Partial’ treatment did not allow root penetration, the availability of diffusion limited nutrients (like P) should be similar in the ‘Partial’ and ‘Isolated’ treatments; but the soil solution could pass through the mesh so that the roots might be able to respond to their neighbours if there was transmission of soluble signalling compounds between neighbouring plants. The mesh in the PC treatment could also allow root hairs or mycorrhizal hyphae to pass.
Two P treatments were applied: a control treatment (HP) received P at the rate of 42 mg kg–1 P as triple super phosphate fertiliser (0–46–0) and a low P treatment (LP) received only half this concentration of P (21 mg kg–1). Nitrogen and potassium were supplied as ammonium nitrate and potassium sulfate at 115 and 104 mg kg–1 soil N and K2O respectively. All nutrients were thoroughly mixed into the media.
Plant growth
The growth medium consisted of 55% sand, 40% coarse vermiculite and 5% red soil (as described above). In each pot, a 24 cm diameter piece of 2 mm nylon mesh was placed at a depth of 7 cm to maintain the basic architecture of the root system when the media was washed away at harvest (Fig. 1). When filling pots for the ‘Partial’ and ‘Isolated’ treatments, a rigid plastic sheet was used to support the barriers while the pot was filled to ensure that volume on both sides remained equal. Germination of seeds was as described above, but the genotype of common bean was a different member of the same RIL population (RIL 13 from the L88 population). Two germinated seeds were planted in each pot at a depth of 4 cm. Plants were irrigated twice daily with 100 mL of nutrient solution and allowed to drain freely. The nutrient solution contained (in addition to the N,P and K concentrations listed above): 2 mM Ca, 2 mM SO4, 0.5 mM Mg, 0.05 mM Cl, 2.5 µM Fe-EDTA, 1.25 µM B, 1 µM Mn, 1 µM Zn, 0.25 µM Mo and 0.25 µM Cu. Nutrient solution pH was adjusted to 5.5–5.8. Plants were grown between June and July 2007 in the greenhouse described above, with an average temperature of 26°C, ranging from 30°C at day to 23°C at night. The photoperiod was 14/10 h (day/night) and the maximum midday photosynthetic flux density was 1400 µmol photons m–2 s–1.
Biomass, P and root measurement
Plants were harvested at 15 and 30 DAP and the DW of the shoots were measured after drying at 60°C for 48 h. The basal root angle for each basal root was measured 15 DAP. The roots above the nylon mesh were cut and washed from the media carefully and the locations where the taproot and basal roots (for each plant) passed through the nylon mesh were individually marked. The distances between basal root and taproot interception of the screen and the height of the basal root origin above the nylon mesh were measured and the basal root angle for each basal root was estimated using from the ratio of these distances – the tangent (Fig. 1c). Root length and root biomass were determined as described in the first experiment at 15 and 30 DAP, but were determined separately for the surface horizon (above the nylon screen) and the deeper horizon. At second harvest (30 DAP) the plants had already developed adventitious roots which were included in root tallies for each region.
The soil in each pot was divided between the two plants along the plane where the barriers were installed. Each half was divided into two regions: the ‘towards’ region, which was nearest the neighbour and the ‘away’ region, which was furthest from the neighbour (Fig. 1b). The horizontal distribution of basal roots in two regions of each pot, the ‘towards’ and ‘away’ regions, was characterised by counting the number of basal roots in each region. Representative subsamples of root tissue were scanned and the resulting images analysed as detailed above and dried at 60°C for two days. Tissue P content was analysed as described above.
Statistical analysis
All treatments had four replicates. The data were analysed in SAS version 6.12 (SAS Institute, Cary, NC, USA). Under a given main factor, such as P application level, the mean for each neighbour treatment was compared and the main factors subsequently were tested for significant difference using Tukey’s HSD. All interactions were included and non-significant interactions were dropped. Pairwise comparisons were conducted to analysed root architectural parameters, but some pairs of treatments were not tested because the parameters were not measured, for instance basal root angle at 30 DAP, shallow root fraction at 15 DAP.
Results
Experiment 1: Effects of P dose, P concentration and neighbour
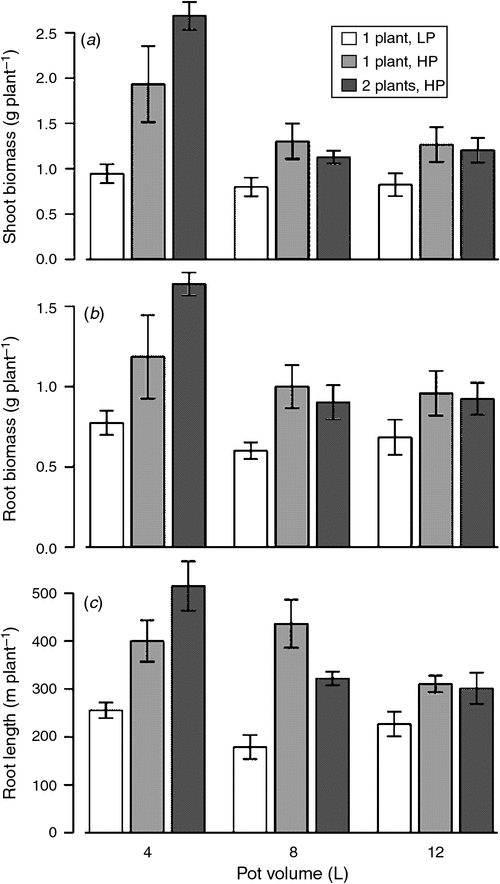
Plant biomass was affected by P availability rather than the presence of a neighbour. Plants receiving the double dosage of P produced more root and shoot biomass (P = 0.0004 root, P = 0.0002 shoot) across all treatment combinations. The greatest biomass was produced by plants receiving the largest amount of P in the smallest volume of soil (i.e. the greatest P concentration; Fig. 2a, b). Even when the double dose of P was diluted in a triple volume of soil, there was sufficient P available to allow plants with neighbours to reach root and shoot biomass comparable to plants with no neighbour (rightmost two bars in Fig. 2).
|
Comparing the one plant with a single dose of P (LP) treatment with the one plant with a double dose of P (HP) treatment shows that the amount of P added was strongly and positively correlated with shoot and root biomass (Fig. 2; Table 2). Furthermore, root length was affected by doubling the quantity of P only in the smaller (4 and 8 L) volumes. Shoot and root biomass and root length in all treatments increased with and was well correlated with the soil P concentration (Table 2).
The effect of a neighbour can be tested in two different ways: comparing one plant with two plants with the same quantity of soil P (one plant HP vs two plants HP) and by comparing one plant with two plants with the same quantity of P per plant (one plant LP vs two plants HP). For the first test, the neighbour did not alter shoot or root biomass and affected root length differently depending on pot volume (Fig. 2, light grey vs dark grey bars; Table 2). For the second test, where both number of plants and quantity of P were varied, the effect of a neighbour was significant and was greatest in the smallest volumes for shoot and root biomasses as well as for root length (Fig. 2, white vs dark grey bars; Table 2). However, in this test the presence of a neighbour is confounded with the quantity of P, which has strong effects on plant growth (Table 2). The presence of a neighbour was not a significant predictor of root or shoot biomass or of root length when comparing single plants with double plants with double volume and the same P concentration (Table 2).
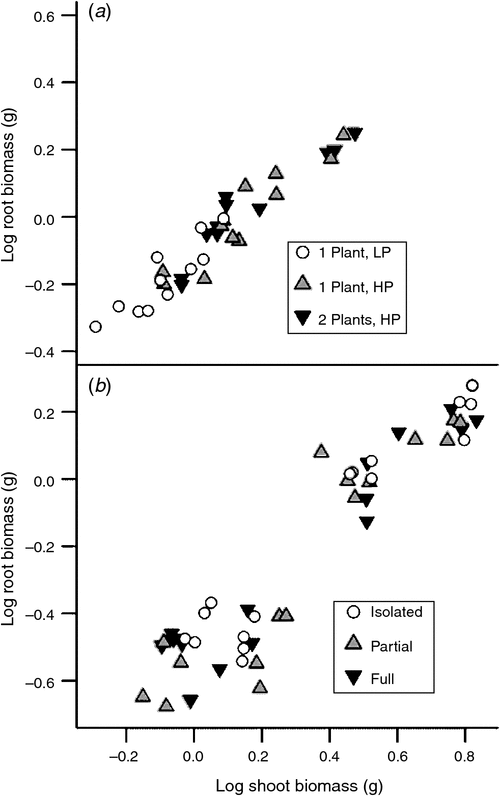
Biomass allocation between shoots and roots, as reflected by the allometric partitioning coefficient (the slope of the relationship between the logarithms of shoot and root biomass), was not affected by either quantity of soil P (P = 0.770) or presence of a neighbour (P = 0.378) (Fig. 5a).
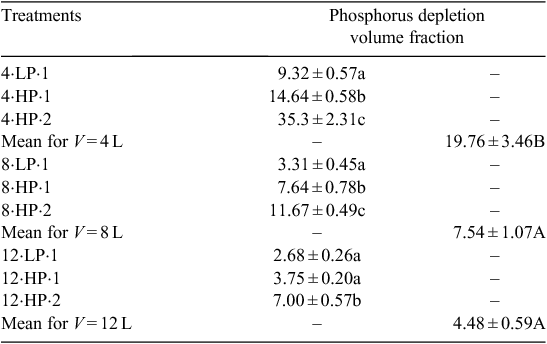
Roots depleted a greater fraction of soil P (estimated from depletion zone volumes) in smaller soil volumes (Table 3). High P increased the depletion zone fraction in the 4 and 8 L soil volumes, but not in the 12 L volume.
|
Experiment 2: Effects of P stress and neighbour
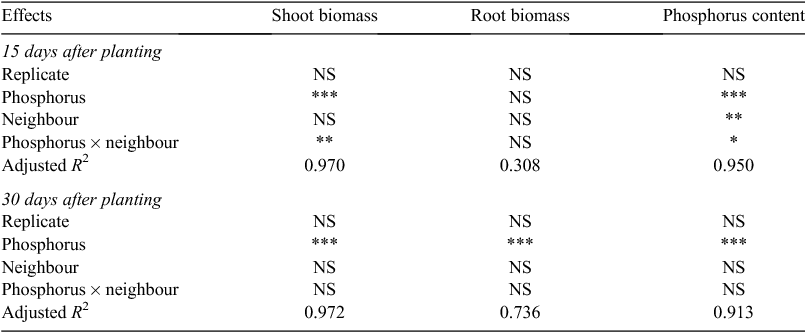
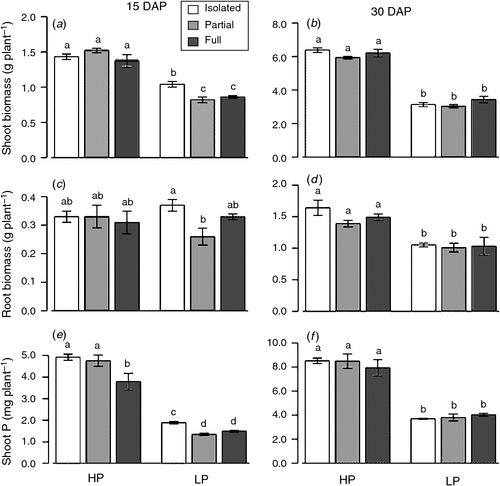
Low P concentration reduced shoot biomass at both harvests and root biomass at the second harvest. However, the allometric partitioning coefficient was constant, indicating that P availability altered the overall growth rate, but not the allocation pattern (Fig. 5b). The neighbour treatments did not affect either root or shoot biomass (Fig. 3; Table A1). Both neighbour and P addition affected shoot P content at 15 DAP but only P addition significantly altered shoot P content at 30 DAP (Table A1). Phosphorus content of plants with full access to the neighbour was less than that of plants with no neighbour at 15 DAP (Fig. 3e).
|
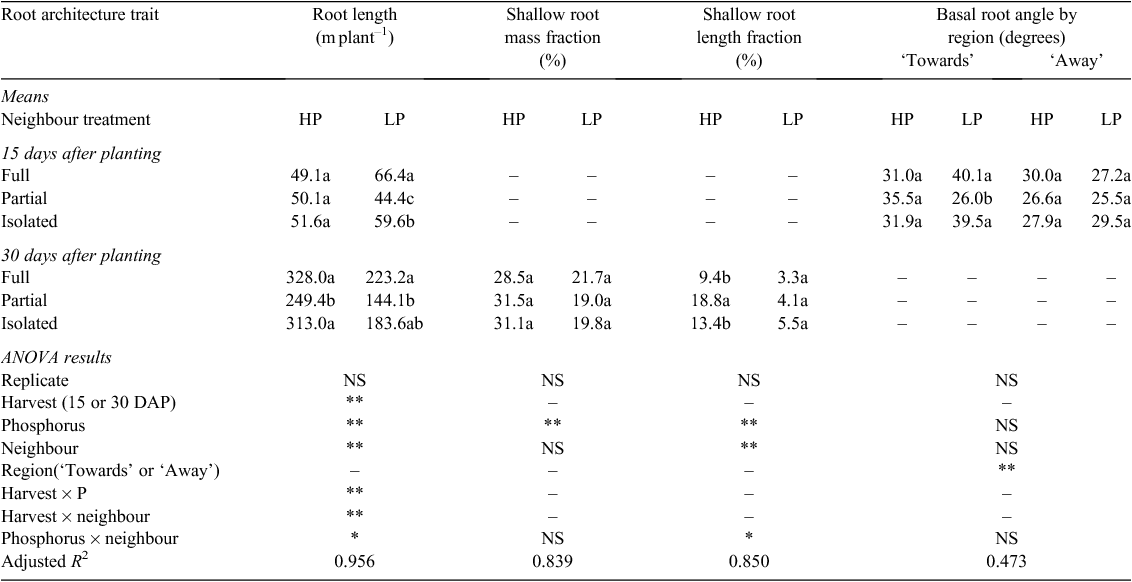
Phosphorus availability significantly altered root length and root architecture. Under Low P, full access to the neighbour increased root length compared with no access to the neighbour at 15 DAP but this difference was not evident at 30 DAP. Low P significantly reduced the shallow fraction of both root mass and length, whereas full access to the neighbour reduced the shallow root length fraction only (Table 4).
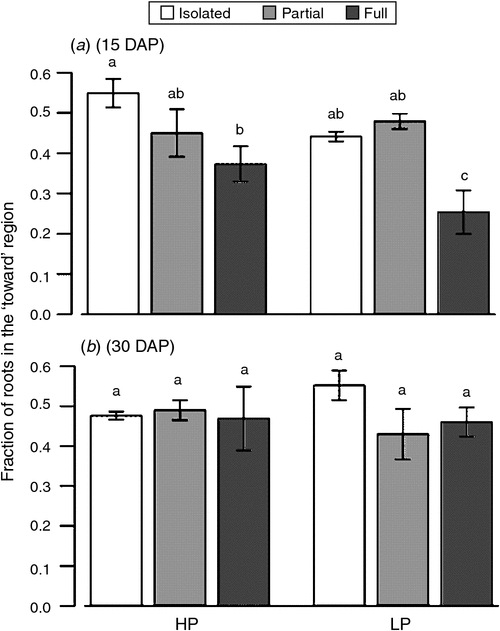
Overall, neighbour treatments did not alter the angles of the basal roots, although the angle of the basal roots differed slightly between ‘towards’ and ‘away’ regions (Table 4). At 15 DAP, the basal roots in the ‘towards’ region had steeper (deeper) angles than roots in ‘away’ region across all neighbour and P level combinations. Low P tended to increase (steepen) the basal root angles in the ‘towards’ region in full- and no neighbour, but this trend was not significant. The radial distribution of basal roots at 15 DAP was also affected by the neighbour treatments. Plants having full access to the neighbour positioned fewer roots in the ‘towards’ region (adjacent to the neighbouring plant), compared with plants with no access to the neighbour (Fig. 4). By 30 DAP radial root deployment was equivalent in ‘towards’ and ‘away’ regions.
|
|
Discussion
Phosphorus concentration rather than self/non-self recognition influences root response to the presence of a neighbour
This study was designed to critically test the ROR and the alternative hypothesis that roots respond to resource availability rather than the presence of a competitor. We found no evidence in support of the ROR. The presence of a neighbour did not alter biomass allocation to roots (Fig. 2; Table 2; Table A1) and the comparison of one plant in 4 L of LP media with two plants in 8 L of HP media, which most closely mimics the conditions of the Gersani et al. (2001) experiment, yields no significant effects on shoot or root biomasses and only marginal effects on root length (Table 2). Notably, allometric partitioning between roots and shoots was unaltered by the presence of a neighbour (Fig. 5). However, reduced P availability increased relative biomass allocation to roots (Fig. 3a–d), in agreement with many previous reports (reviewed by Lynch and Ho 2005). These results are consistent with the hypothesis that any root proliferation in response to the presence of a neighbour occurs as a response to resource depletion, rather than as a response to ‘non-self’ roots.
Increasing P concentration significantly affected shoot and root biomasses and had a stronger effect than that of rooting volume or the presence of a neighbour (Table 2). This is consistent with the idea that nutrient availability is one of the driving forces in root competition (Wilson and Tilman 1993). Further, in the smallest soil volume (4 L), where competition for soil resources should be most intense because of greater root length density and overlap of depletion zones (Table 3), we observed no change in either root or shoot biomass when a neighbour was present, as would be predicted by the ROR (Fig. 2).
The degree of resource depletion (of P) should be positively related to root length. When one plant grows in a pot only intraplant competition exists. Enlarging the soil volume should lead to a decrease of both root length density (root length/soil volume) and P depletion zone fraction (Fig. 2c; Table 2). This should allow a decline in the overlap of depletion zones and therefore a reduction in intraplant competition. Whether such a decline in self-competition occurs will depend on the overall root length density and the architecture of the root system. A decline in self-competition would reduce the cost of resource acquisition, potentially leading to an increase in shoot biomass, though such an increase may be small. While we observed no such increase in shoot biomass with increasing rooting volume (Fig. 2), our results are otherwise consistent with the idea that root competition for P (both inter- and intraplant) should increase in smaller rooting volumes, as root length density (root length/volume) and the overlap of P depletion zones, increases (Fig. 2; Table 3).
We note however, that even in the smallest soil volume with two plants, the total estimated depletion zone volume was less than 40% (Table 3) and if we assume a similar range for the ratio of interplant to intraplant competition as reported by Rubio et al. (2001) for plants separated by 6 cm (9.4–35.3%), then the actual volume in which interplant competition would occur is likely less than 5% of the total volume. If the maximum likely volume of interplant competition is this small, interplant competition may be relatively unimportant and the lack of response to a neighbour in the 8 and 12 L pots is expected. It is possible that interplant competition for a more mobile nutrient, like nitrate, would result in different responses to the presence of a neighbour.
Root architecture will exhibit greater plasticity in response to the presence of a neighbour than will root biomass
Most studies that have addressed root competition have investigated the alteration of root biomass and have rarely considered the role root architecture may play in belowground competition. Root architecture has been shown to be an important determinant of P acquisition by increasing foraging efficiency (Lynch 2005). This study analysed root architectural modification in order to critically test the hypothesis that root architecture will exhibit greater plasticity in response to the presence of a neighbour than will root biomass.
Root architectural traits, such as root length and vertical and horizontal distribution, were altered by the presence of a neighbour (Fig. 4; Table 4) even though root biomass was similar across the three neighbour treatments (Fig. 3c, d). Thus, root architecture was modified without alteration of root biomass. Root architectural plasticity may reduce competition for soil resources without altering biomass partitioning between shoot and roots. The presence of a neighbour caused the plants to increase root length (Table 3), place fewer roots in the ‘toward’ region than in the ‘away’ region (Fig. 4) and place fewer roots in the topsoil than in the subsoil (Table 4). These architectural responses may reduce the overlap of P depletion zones thus reducing the intensity of both intraplant and interplant competition. Structural–functional plant models (Ge et al. 2000; Rubio et al. 2001; Postma and Lynch 2011) could be used to estimate the effect of such architectural responses on P acquisition. Thus, root architecture exhibits greater plasticity in response to the presence of a neighbour than root biomass, providing support for the second hypothesis. Changes in root biomass alone may not adequately characterise root responses to a neighbour and root architecture should also be considered.
Conclusion
Root architecture was found to be more sensitive to the presence of a neighbour than root biomass. In these studies, the presence of a neighbour did not alter root biomass, but did alter root architecture and in some cases root length, in a manner that could reduce interplant competition in both vertical and horizontal dimensions. An analogous architectural response occurs in shoot responses to aboveground competition, termed the stress-induced morphogenic response (Potters et al. 2007). Root architecture is regulated by P concentration (Lynch and Brown 2008), including root gravitropic setpoint angle (Bonser et al. 1996), production of shoot-borne roots (Miller et al. 2003; Kim et al. 2008), axial elongation (Ma et al. 2003), lateral branching (Borch et al. 1999; Desnos 2008) and root hair formation (Bates and Lynch. 1996; Ma et al. 2001). Increased axial elongation in response to lower P availability (Ma et al. 2003) could account for some of the increased root length attributed to competition. Results from these studies are consistent with the hypothesis that architectural responses to local P availability are more important than self/non-self recognition or soil volume in root responses to neighbouring plants.
Acknowledgements
The authors thank Robert Snyder and Phanchita Vejchasarn for technical assistance and Johannes Postma and Larry York for their critical comments. This research was supported by the USAID Pulse CRSP and National Science Foundation of China (No. 30890133), ‘The innovative group grant of NSFC’ (No. 30821003).
References
Barber SA (1995) ‘Soil nutrient bioavailability: a mechanistic approach.’ (John Wiley & Sons: New York)Bates TR, Lynch JP (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell & Environment 19, 529–538.
| Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XktlGgt78%3D&md5=5b05551f17a0afb16c465c344e5fbeb0CAS |
Bonser A, Lynch JP, Snapp S (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytologist 132, 281–288.
| Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnlvFOjtg%3D%3D&md5=87d6675e68df47b4ca2c5c8ee3da42e8CAS |
Borch K, Bouma TJ, Lynch JP, Brown KM (1999) Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant, Cell & Environment 22, 425–431.
| Ethylene: a regulator of root architectural responses to soil phosphorus availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjvFShsLo%3D&md5=224baf3c3ca907de809c82d5b78a6897CAS |
Callaway RM, Pennings SC, Richards CL (2003) Phenotypic plasticity and interactions among plants. Ecology 84, 1115–1128.
| Phenotypic plasticity and interactions among plants.Crossref | GoogleScholarGoogle Scholar |
Casper BB, Jackson RB (1997) Plant competition underground. Annual Review of Ecology and Systematics 28, 545–570.
| Plant competition underground.Crossref | GoogleScholarGoogle Scholar |
Chevalier F, Pata M, Nacry P, Doumas P, Rossignol M (2003) Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant, Cell & Environment 26, 1839–1850.
| Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmt1eltQ%3D%3D&md5=0d99b421f3fbb05758d928ea8521f421CAS |
Crawley MJ (2005) ‘Statistics: an introduction using R.’ (John Wiley & Sons: Hoboken, NJ, USA)
de Kroon H, Mommer L, Nishiwaka A (2003) Root competition: towards a mechanistic understanding. In ‘Root competition: toward a mechanistic understanding. Ecological studies. No. 168’. (Eds H de Kroon, EJ Visser) pp. 215–234. (Springer-Verlag: Heidelberg, Germany)
Desnos T (2008) Root branching responses to phosphate and nitrate. Current Opinion in Plant Biology 11, 82–87.
| Root branching responses to phosphate and nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVeqtb8%3D&md5=ebc018685fd806cc3558d9dcbdec594bCAS |
Endlweber K, Scheu S (2006) Effects of Collembola on root properties of two competing ruderal plant species. Soil Biology & Biochemistry 38, 2025–2031.
| Effects of Collembola on root properties of two competing ruderal plant species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XotFyktLg%3D&md5=1619ff33c0f0ee55405072e72aa2facaCAS |
Epstein E (1972) ‘Mineral nutrition of plants: principles and perspectives.’ (John Wiley & Sons: New York)
Falik O, Reides P, Gersani M, Novoplansky A (2003) Self/non-self discrimination in roots. Journal of Ecology 91, 525–531.
| Self/non-self discrimination in roots.Crossref | GoogleScholarGoogle Scholar |
Farley RA, Fitter AH (1999) The responses of seven co-occurring woodland herbaceous perennials to localised nutrient rich patches. Journal of Ecology 87, 849–859.
| The responses of seven co-occurring woodland herbaceous perennials to localised nutrient rich patches.Crossref | GoogleScholarGoogle Scholar |
Gahoonia TS, Nielsen NE (1992) The effects of root-induced pH changes on the depletion of inorganic and organic phosphorus in rhizosphere. Plant and Soil 143, 185–191.
| The effects of root-induced pH changes on the depletion of inorganic and organic phosphorus in rhizosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XltlSnsb0%3D&md5=fce87066db4b5c61918c7e903441a0f1CAS |
Ge ZY, Rubio G, Lynch JP (2000) The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model. Plant and Soil 218, 159–171.
| The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhvVGrtLc%3D&md5=4a1f1cc5f369741441e0cdfae1a5093bCAS |
Gersani M, Brown JS, O’Brien EE, Maina GM, Abramsky A (2001) Tragedy of the commons as a result of root competition. Journal of Ecology 89, 660–669.
| Tragedy of the commons as a result of root competition.Crossref | GoogleScholarGoogle Scholar |
Goldberg DE, Barton AN (1992) Patterns and consequences of interspecific competition in natural communities: a review of field experiments with plants. American Naturalist 139, 771–801.
| Patterns and consequences of interspecific competition in natural communities: a review of field experiments with plants.Crossref | GoogleScholarGoogle Scholar |
Gruntman M, Novoplansky A (2004) Physiologically mediated self/non-self discrimination in roots. Proceedings of the National Academy of Sciences of the United States of America 101, 3863–3867.
| Physiologically mediated self/non-self discrimination in roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXis1Kgurk%3D&md5=c7aa15c8726a8ecbf31cd47e598e8fe4CAS |
Hess L, de Kroon H (2007) Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination. Journal of Ecology 95, 241–251.
| Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination.Crossref | GoogleScholarGoogle Scholar |
Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW (2005) Rhizosphere geometry and heterogeneity arising from root mediated physical and chemical processes. New Phytologist 168, 293–303.
| Rhizosphere geometry and heterogeneity arising from root mediated physical and chemical processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Smu7bJ&md5=97c77935f1df23a88d9fcdc11218a5c4CAS |
Ho MD, Ross JC, Brown KM, Lynch JP (2005) Root architectural tradeoffs for water and phosphorus acquisition. Functional Plant Biology 32, 737–748.
| Root architectural tradeoffs for water and phosphorus acquisition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmvVOgsLw%3D&md5=e3114c94dd42899633801a7154e62879CAS |
Holzapfel C, Alpert P (2003) Root cooperation in a clonal plant: connected strawberries segregate roots. Oecologia 134, 72–77.
| Root cooperation in a clonal plant: connected strawberries segregate roots.Crossref | GoogleScholarGoogle Scholar |
Jiang CF, Gao XH, Liao LL, Harberd NP, Fu XD (2007) Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signalling pathway in Arabidopsis. Plant Physiology 145, 1460–1470.
| Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signalling pathway in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVCntb7I&md5=126436af538361cc620d28c236177193CAS |
Kim HJ, Lynch JP, Brown KM (2008) Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant, Cell & Environment 31, 1744–1755.
| Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmt1yrug%3D%3D&md5=f8e85709144dc6823890140331f81e95CAS |
Liao H, Rubio G, Yan XL, Cao XL, Brown KM, Lynch JP (2001) Effect of phosphorus availability on basal root shallowness in common bean. Plant and Soil 232, 69–79.
| Effect of phosphorus availability on basal root shallowness in common bean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsVCks7s%3D&md5=27107da23ed671c3fb0751e679cefa63CAS |
Linkohr BI, Wlliamson LC, Fitter AH, Leyser HMO (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. The Plant Journal 29, 751–760.
| Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xjs12msr8%3D&md5=8ad67e21fc416e19a7c82f30a4511dffCAS |
Lynch JP (1995) Root architecture and plant productivity. Plant Physiology 109, 7–13.
Lynch JP (2005) Root architecture and nutrient acquisition. In ‘Nutrient acquisition by plants: an ecological perspective. Ecological Studies. No. 181’. (Ed. H BassiriRad) pp. 146–183. (Springer-Verlag: Heidelberg, Germany)
Lynch JP, Brown KM (2001) Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil 237, 225–237.
| Topsoil foraging – an architectural adaptation of plants to low phosphorus availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XovVWltA%3D%3D&md5=71bc932d0f826182691b51c3a1545899CAS |
Lynch JP, Brown KM (2006) Whole plant adaptations to low phosphorus availability. In ‘Plant–environment interactions’. 3rd edn. (Ed. B Huang) pp. 209–242. (CRC Press: Boca Raton, FL, USA)
Lynch JP, Brown KM (2008) Root strategies for P acquisition. In ‘The ecophysiology of plant–phosphorus interactions’. (Eds PJ White, JP Hammond) pp. 83–116. (Springer: Dordrecht, The Netherlands)
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant and Soil 269, 45–56.
| Rhizoeconomics: carbon costs of phosphorus acquisition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXks1Oisrw%3D&md5=9e5b322478004d2cfd7791f08b5f391bCAS |
Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant, Cell & Environment 24, 459–467.
| Regulation of root hair density by phosphorus availability in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtFyjtb0%3D&md5=911617b14cd01c2b3bfd6ec01659f33fCAS |
Ma Z, Baskin TI, Brown KM, Lynch JP (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiology 131, 1381–1390.
| Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXisFels7c%3D&md5=f9237b297fce4413ccd1a2cf0bd74997CAS |
Matthes-Sears U, Larson DW (1999) Limitations to seedling growth and survival by the dose and quality of rooting space: implications for the establishment of Thuja occidentalis on cliff faces. International Journal of Plant Sciences 160, 122–128.
| Limitations to seedling growth and survival by the dose and quality of rooting space: implications for the establishment of Thuja occidentalis on cliff faces.Crossref | GoogleScholarGoogle Scholar |
McConnaughay KDM, Bazzaz FA (1991) Is physical space a soil resource? Ecology 72, 94–103.
| Is physical space a soil resource?Crossref | GoogleScholarGoogle Scholar |
Miller CR, Ochoa I, Nielsen KL, Beck D, Lynch JP (2003) Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Functional Plant Biology 30, 973–985.
| Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsVWnsrk%3D&md5=98a85d3b0efe3c84a32f3aa436b6aaaeCAS |
Moore PD (2003) Roots of diversity. Nature 424, 26–27.
| Roots of diversity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltVels74%3D&md5=cbde01a414e99a032459622a665808e2CAS |
Murphy GP, Dudley SA (2007) Above- and belowground competition cues elicit independent responses. Journal of Ecology 95, 261–272.
| Above- and belowground competition cues elicit independent responses.Crossref | GoogleScholarGoogle Scholar |
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36.
| A modified single solution method for the determination of phosphate in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF38XksVyntr8%3D&md5=5af6c72dbb34afeb0425d69536a675b9CAS |
Niklas KJ, Enquist BJ (2002) On the vegetative biomass partitioning of seed plant leaves, stems and roots American Naturalist 159, 482–497.
O’Brien EE, Brown JS (2008) Games roots play: effects of soil volume and nutrients. Journal of Ecology 96, 438–446.
Passarge J, Hol S, Escher M, Huisman J (2006) Competition for nutrients and light: stable coexistence alterative stable states, or competitive exclusion? Ecological Monographs 76, 57–72.
| Competition for nutrients and light: stable coexistence alterative stable states, or competitive exclusion?Crossref | GoogleScholarGoogle Scholar |
Pinheiro J, Bates D, DebRoy S, Sarkar D (2009) ‘nlme: Linear and nonlinear mixed effects models. R package ver. 3.1–96.’ Available at http://www.R-project.org [Verified 28 September 2011]
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK (2007) Stress-induced morphogenic responses: growing out of trouble? Trends in Plant Science 12, 98–105.
| Stress-induced morphogenic responses: growing out of trouble?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXivVShtro%3D&md5=fb02d16c7908c957db9cbce58863bf82CAS |
Postma JA, Lynch JP (2011) Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Annals of Botany 107, 829–841.
| Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvFahs70%3D&md5=e942a0f9f56da67b0a9b2f43559cee46CAS |
R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org [Verified 28 September 2011]
Rubio G, Walk T, Ge ZY, Yan XY, Liao H, Lynch JP (2001) Root gravitropism and belowground competition among neighbouring plants: a modelling approach. Annals of Botany 88, 929–940.
| Root gravitropism and belowground competition among neighbouring plants: a modelling approach.Crossref | GoogleScholarGoogle Scholar |
Rubio G, Liao H, Yan XL, Lynch JP (2003) Topsoil foraging and its role in plant competitiveness for phosphorus in common bean. Crop Science 43, 598–607.
| Topsoil foraging and its role in plant competitiveness for phosphorus in common bean.Crossref | GoogleScholarGoogle Scholar |
Schenk HJ (2006) Root competition: beyond resource depletion. Journal of Ecology 94, 725–739.
| Root competition: beyond resource depletion.Crossref | GoogleScholarGoogle Scholar |
Semchenko M, Hutchings MJ, John EA (2007) Challenging the tragedy of the commons in root competition: confounding effects of neighbour presence and substrate volume. Journal of Ecology 95, 252–260.
| Challenging the tragedy of the commons in root competition: confounding effects of neighbour presence and substrate volume.Crossref | GoogleScholarGoogle Scholar |
Walk TC, van Erp E, Lynch JP (2004) Modelling applicability of fractal analysis to efficiency of soil exploration by roots. Annals of Botany 94, 119–128.
| Modelling applicability of fractal analysis to efficiency of soil exploration by roots.Crossref | GoogleScholarGoogle Scholar |
Walk TC, Jaramillo R, Lynch JP (2006) Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant and Soil 279, 347–366.
| Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhsVarur4%3D&md5=91a31676a808428094a37ea19b48d9e3CAS |
Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiology 126, 875–882.
| Phosphate availability regulates root system architecture in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXks1GmsLs%3D&md5=49a3cd31dddaf841f767c96e2d8cb4baCAS |
Wilson S, Tilman D (1993) Plant competition and resource availability in response to disturbance and fertilisation. Ecology 74, 599–611.
| Plant competition and resource availability in response to disturbance and fertilisation.Crossref | GoogleScholarGoogle Scholar |
Wilson S, Tilman D (2002) Quadratic variation in old-field species richness along gradients of disturbance and nitrogen. Ecology 83, 492–504.
| Quadratic variation in old-field species richness along gradients of disturbance and nitrogen.Crossref | GoogleScholarGoogle Scholar |
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) ‘Mixed effects models and extensions in ecology with R.’ (Springer-Verlag: New York)