The role of leaf hydraulic conductance dynamics on the timing of leaf senescence
Juan Pablo Giraldo A B , James K. Wheeler A , Brett A. Huggett A and N. Michele Holbrook AA Harvard University, Department of Organismic and Evolutionary Biology, 16 Divinity Avenue, Cambridge, MA 02138, USA.
B Corresponding author. Email: jgiraldo@post.harvard.edu
Functional Plant Biology 41(1) 37-47 https://doi.org/10.1071/FP13033
Submitted: 10 February 2013 Accepted: 27 June 2013 Published: 14 August 2013
Abstract
We tested the hypothesis that an age-dependent reduction in leaf hydraulic conductance (Kleaf) influences the timing of leaf senescence via limitation of the stomatal aperture on xylem compound delivery to leaves of tomato (Solanum lycopersicum L.), the tropical trees Anacardium excelsum Kunth, Pittoniotis trichantha Griseb, and the temperate trees Acer saccharum Marsh. and Quercus rubra L. The onset of leaf senescence was preceded by a decline in Kleaf in tomato and the tropical trees, but not in the temperate trees. Age-dependent changes in Kleaf in tomato were driven by a reduction in leaf vein density without a proportional increase in the xylem hydraulic supply. A decline in stomatal conductance accompanied Kleaf reduction with age in tomato but not in tropical and temperate tree species. Experimental manipulations that reduce the flow of xylem-transported compounds into leaves with open stomata induced early leaf senescence in tomato and A. excelsum, but not in P. trichantha, A. saccharum and Q. rubra leaves. We propose that in tomato, a reduction in Kleaf limits the delivery of xylem-transported compounds into the leaves, thus making them vulnerable to senescence. In the tropical evergreen tree A. excelsum, xylem-transported compounds may play a role in signalling the timing of senescence but are not under leaf hydraulic regulation; leaf senescence in the deciduous trees A. trichanta, A. saccharum and Q. rubra is not influenced by leaf vascular transport.
Additional keywords: chlorophyll, leaf anatomy, leaf phenology, photosynthesis, tomato, vascular transport.
Introduction
Leaf senescence is a key developmental transition in which chlorophyll, cytoplasmic proteins and cellular membranes are degraded, and nutrients are remobilised from senescing leaves to young leaves, seeds, fruits, storage tissues and new buds (Himelblau and Amasino 2001). It has been proposed that leaf senescence functions to optimise plant nitrogen distribution (Ono et al. 2001); to reduce water use, thus facilitating plant survival under drought conditions; and to boost plant fitness by accelerating the reproductive output in monocarpic plants under water stress (Munné-Bosch and Alegre 2004). Despite its importance, it is not known how plants determine the onset of leaf senescence (Lim et al. 2007). Studies in a tropical moist forest of Panama demonstrate that leaf fall is not determined by soil water availability, suggesting instead that vapour pressure deficit (VPD) or irradiance trigger leaf abscission (Wright and Cornejo 1990; Wright 1991; Wright and Vanschaik 1994). Temperature and photoperiod have been commonly identified as environmental drivers of leaf senescence in temperate forests but hypotheses to explain the underlying physiological mechanisms lack experimental evidence (Estrella and Menzel 2006).
The marked changes in expression of senescence-associated genes (SAGs) that accompany the onset of senescence in herbaceous plants indicate that it is a highly regulated developmental transition (Buchanan-Wollaston et al. 2003; Guo and Gan 2005; Lim et al. 2007). SAG expression is an integrated response of developmental age and environmental stressors such as UV-B, ozone, nutrient limitation, temperature, shading, pathogens and phytohormones (Lim et al. 2007). A decline in photosynthesis has been proposed as a signal for age-dependent leaf senescence (Hensel et al. 1993) but the evidence remains controversial (Guo and Gan 2005). In Arabidopsis thaliana (L.) Heynh. mutants and transgenic tobacco (Nicotiana tabacum L.) plants with reduced photosynthetic activity, age-dependent leaf senescence is delayed compared with wild-type plants (Miller et al. 2000; Woo et al. 2002). Opposing hypotheses positing that sugar starvation or sugar accumulation in leaf cells initiate senescence lack decisive evidence (van Doorn 2008). To date, how the developmental age of leaves is recognised during senescence and the nature of the signal is poorly understood.
The plant vascular system, as the nexus of hormone and nutrient transport, is well positioned to act as an age-dependent signalling system controlling leaf senescence. It is sensitive to a wide range of physiological (water and nutrient availability), environmental (light and temperature) and age-related factors identified as inducing leaf senescence (Sack and Holbrook 2006; Lim et al. 2007). An age-dependent decline in the conductance of the liquid phase of water through leaves (leaf hydraulic conductance (Kleaf); mmol m–2 s–1 MPa–1) was associated with the onset of leaf fall (Salleo et al. 2002; Brodribb and Holbrook 2003a; Lo Gullo et al. 2004) but a causal role for Kleaf in leaf senescence was not proposed. Because Kleaf is often a major bottleneck in the movement of water through plants (Sack and Holbrook 2006), it is the most likely component of the vascular system to act as a hydraulic regulator of leaf senescence. Through its effect on leaf water status, Kleaf can limit stomatal aperture (Meinzer 2002; Brodribb and Holbrook 2003b), thus influencing the delivery of key xylem-transported regulators of leaf senescence.
Both cytokinins and nitrogen compounds are transported by the xylem and have been identified to play a role in leaf senescence (Pourtau et al. 2004; Boonman et al. 2007). Cytokinins are well known to delay leaf senescence and even cause regreening of yellowing leaves of species such as A. thaliana, tomato (Solanum lycopersicum L.) and tobacco (Richmond and Lang 1957; Gan and Amasino 1995; Gan and Amasino 1996; Lo Gullo et al. 2004; Guo and Gan 2005). In A. thaliana, experimental manipulations that reduce transpiration but not stomatal conductance induce senescence, with decreased cytokinin delivery implicated as the driver of leaf chlorophyll degradation (Boonman et al. 2007). Soil nitrogen deficiency in herbaceous species leads to sugar accumulation in leaves (Ono and Watanabe 1997), which, in turn, triggers the expression of SAGs (Rolland et al. 2006). The highly specific SAG12 encoding a cysteine protease can be induced by 100-fold during leaf senescence by growing A. thaliana plants in 2% glucose in combination with low nitrogen availability (Pourtau et al. 2004; Wingler et al. 2004).
Kleaf reduction with leaf age has been reported in tropical and temperate trees (Salleo et al. 2002; Brodribb and Holbrook 2003a) and herbaceous plants (Lo Gullo et al. 2004), although whether this is due to changes in the leaf xylem or mesophyll hydraulic pathways remains unclear. A possible explanation for Kleaf reduction with leaf age is that the xylem’s structural components deteriorate, thus impairing leaf function. For example, degradation of pit membranes can make aging vessels more prone to air-seeded embolisms by lowering the pressure threshold required for embolism formation (Sperry et al. 1991). Alternatively, the reduction of Kleaf with age could take place in the extra-xylary pathways, where water may encounter most of the hydraulic resistance within leaves (Nardini and Salleo 2005; Brodribb et al. 2007). Changes in protoplast and parenchymal wall properties have been implicated in the ontogenetic reduction of mesophyll conductance (Aasamaa et al. 2005). Simple thickening of the cell walls in the apoplastic pathway or structural changes in the plasmodesmata through the symplast could significantly influence Kleaf. Age-related accumulation of the phloem’s immobile compounds in leaf cell membranes such as calcium (McLaughlin and Wimmer 1999) may contribute to reduce cell membrane permeability via downregulation of aquaporin conductance (Kaldenhoff and Fischer 2006; Kaldenhoff et al. 2008).
In this study, we examined the hypothesis that a decline in Kleaf as leaves age influences the onset of leaf senescence via limitation of the stomatal aperture on xylem compound delivery to the leaves of tomato plants, and tropical and temperate tree species. We first conducted a comprehensive study of greenhouse tomato plants to test our hypothesis under controlled laboratory conditions. We then scaled up our experiments to more challenging field conditions in the canopy of tropical and temperate trees. To test our hypothesis we determined (1) whether the Kleaf decline precedes a significant reduction in leaf chlorophyll content at the onset of leaf senescence; (2) if the Kleaf drop limits gas exchange, thus impacting leaf xylem compound delivery into leaves; and (3) whether downregulating the flow of xylem-transported compounds into leaves, through a reduction in transpiration rates with open stomata, induces earlier leaf senescence.
Materials and methods
Plant material
Wild-type glasshouse tomato plants (Solanum lycopersicum L. var. MP-1) were sampled when plants were 2–3 months old and had at least 16 nodes. Tomato plants were grown in 4-L pots with a soil mixture of Fafard Mix#3B (Sungro, Agawan, MA, USA). Soil was irrigated to field capacity twice a day at 0800 hours and 1400 hours. Plants were maintained at glasshouse conditions of 25°C day : 19°C night during a 13-h photoperiod. A set of five plants was used to determine the relationship between average leaf age and position (leaf node from apex, LNA), where leaf age (days) = 2.33 × LNA – 2.94 (R2 = 0.9694, P < 0.001; n = 5). In tropical and temperate trees, several leaves per individual were measured monthly from expanded to presenesced leaves on randomly selected sun branches. Three tropical canopy trees of Anacardium excelsum Kunth and Pittoniotis trichantha Griseb up to 45 m in height were accessed using the Smithsonian Tropical Research Institute crane located in Parque Nacional Metropolitano, Panama. Young leaves were fully expanded and labelled at the beginning of the dry season in November 2008. Field experiments in temperate trees were performed at Harvard Forest, Petersham, Massachusetts, during the summers of 2008 and 2009 on three individuals each of the deciduous tree species Acer saccharum Marsh. (sugar maple) and Quercus rubra L. (red oak). Individuals were distributed along a road near the main laboratory facilities, and were accessed with a ladder and the Harvard Forest canopy lift. Young leaves completed expansion in mid- to late June, and were shed in September and October in A. saccharum and Q. rubra, respectively. Leaf age was calculated as the number of days from the date of completed leaf expansion.
Leaf chlorophyll content and senescence
A significant decline in leaf chlorophyll content was used as an indicator of the onset of leaf senescence (Fig. S1, available as Supplementary Material to this paper). Chlorophyll degradation has been reported to precede the remobilisation of nitrogen from chlorophyll-binding proteins (Hörtensteiner 2006) and dismantling of thylakoid membrane proteins (Thomas et al. 2002). The expression of the senescence-specific gene SAG12 in tomato leaves has been associated with a significantly lower chlorophyll content compared with young leaves (Swartzberg et al. 2006). Leaf chlorophyll absorbance was measured in situ with a chlorophyll meter (SPAD 502, Minolta, Warrington, UK). To calibrate the SPAD readings, total chlorophyll content (a + b) was measured by spectrophotometry (Lee et al. 2003). Linear regressions were used to convert SPAD chlorophyll absorbance measurements to chlorophyll a + b content (µg cm–2) (Fig. S1).
Leaf hydraulic conductance
Water flow through leaves was measured using a modified evaporative flux method (Sack et al. 2002; Brodribb and Holbrook 2006). Petioles of leaves were submerged and cut under water to prevent the formation of embolisms. The base of the petiole was immediately connected via silicone tubing to a flow meter consisting of polyether ether ketone (PEEK) tubing (McMaster-Carr, Robbinsville, NJ, USA) with a known conductance (K) in which the pressure drop (ΔP) was continuously monitored in LABVIEW (National Instruments, Austin, TX, USA) with a pressure transducer (PX26, Omega, Stamford, CT, USA). PEEK tubing flow (F) was calculated by an Ohm’s law analogy as F = K × ΔP. Leaf flow was determined by applying the principle that two conductors connected in series have equal flows. Leaf transpiration was induced with a 500-W halogen lamp (Bayco, Wylie, TX, USA) providing a PAR of 900–1300 µmol m–2 s–1 (measured using a LI-250 light meter (LI-COR, Lincoln, NE, USA). A cooling fan was used to reduce the leaf boundary layer, and a water bath was placed between the leaf and the halogen lamp to prevent overheating. Leaf temperatures measured with an infrared sensor (OS543, Omega) on three leaflets per leaf ranged from 26°C to 32°C with a maximum temperature difference between leaflets of 4°C. Ambient relative humidity near the leaf surface varied from 25% to 60% (SAM990DW digital sling psychrometer, McMaster-Carr). A steady flow was usually reached no earlier than 45 min after connecting the leaves to the water supply and exposing them to the environmental conditions generated by the fan and the halogen lamp. Some leaves were preadapted to these conditions in a replica set up to optimise the use of the flowmeter. Flow values were recorded when the variation was less than 5% of the mean. Leaf water potential was measured with a Scholander pressure chamber (Soil Moisture Equipment Corp., Santa Barbara, CA, USA). Flows were normalised by leaf area determined with a LI-300 leaf area meter (LI-COR). Kleaf, as determined by the evaporative flux method, was calculated using Eqn 1:

Prior to measurements, the flowmeter and an intravenous bag that served as a water reservoir were bleached to prevent bacteria growth, rinsed and filled with deionised and degassed water filtered with a 0.2 µm mesh (Acrodisc syringe filter, Pall, Ann Arbor, MI, USA).
The hydraulic conductance of the most apical tomato leaflet (Kleaflet) was measured by the evaporative flux method as described above (Sack et al. 2002; Brodribb and Holbrook 2006) and leaf-specific rachis hydraulic conductance was determined with a flow meter (Brodribb and Holbrook 2006). The most basal segment of the rachis between the main plant axis and the first petiolule (hereafter referred to as the petiole) was excised underwater with a clean razor blade. All leaflets were excised with only the rachis remaining. Leaf-specific rachis hydraulic conductance was calculated as Krachis = flow ÷ driving pressure, normalised by leaf area supplied by the rachis (measured using a LI-1300 meter (LI-COR)).
Tomato leaf anatomy
Vein density measurements were performed by sampling leaf discs 8 mm in diameter from the central portion of the terminal leaflet of each LNA. Procedures for clearing tissues were based on methods described by Berlyn and Miksche (1976) and Scoffoni and Sack (2010). Samples were imaged at 4× magnification on an Olympus BH2 (Olympus America Inc., Center Valley, PA, USA) equipped with an AxioCam HRc camera (Carl Zeiss MicroImaging, LLC, Thornwood, NY, USA). Images were analysed using ImageJ software (National Institute of Health, Bethesda, MD, USA) with vein density (2° and higher) calculated as total vein length per sample area (mm mm–2).
Petiole xylem area was measured on cross-sections stained with a solution of 95% alcoholic phloroglucinol plus HCl. Petiole segments ~0.5 mm in length were cut near the most proximal leaflet then placed on a microtome (Reichert, Depew, NY, USA), submerged in embedding medium (OCT compound 4583, Tissue Tek, Sakura Finetek, Torrance, CA, USA), frozen and sectioned at 80 µm thickness. Sections were mounted on slides with phloroglucinol for 20 min before imaging at 3× using a digital camera (Axiocam HRc, Zeiss, Thornwood, NY, USA) attached to a dissecting scope (SZ60, Olympus). Petiole xylem area was manually selected from stained regions and area measured in ImageJ (National Institute of Health, Bethesda, MD, USA).
To determine the size distribution of conductive vessels in the petiole of young and old leaves, we performed leaf live staining with acid fuchsin. Petiole sections were observed using a compound microscope (BH-2, Olympus) and an image taken of the third xylem quadrant starting from the top of the petiole in a clockwise direction, with a digital camera (Axiocam HRc, Zeiss) at 10× magnification. Vessels were outlined in ImageJ (NIH) and vessel cell wall traces were measured for area, perimeter, and major and minor axis dimensions.
Leaf gas exchange
Maximum assimilation (A) and stomatal conductance (gs) measurements were performed with a LI-6400 meter (LI-COR). Plants were measured on sunny mornings from 0900 hours to 1200 hours. Leaves of different ages were measured in a random order over the sampling interval to avoid any systematic effect associated with recording time. In the glasshouse, leaf chamber settings were light saturating levels of 1200 μmol m–2 s–1, ambient relative humidity, a block temperature of 25°C, a flow rate of 500 μmol s–1 and average CO2 of 375 ± 5 mol CO2 m–2 s–1. At the tropical site, leaf chamber PAR was set according to light curves, which demonstrated that 1800 μmol m–2 s–1 with 10% blue light was saturating. Reference air CO2 levels in the chamber were regulated with soda lime to 370 ± 5 parts per million. Relative humidity was allowed to vary in the natural range, block temperature was maintained at 30°C and flow rates were set at 500 μmol s–1. At the temperate site, a LI-COR cuvette was set up at a saturating PAR of 1500 μmol m–2 s–1, flow rates of 500 μmol s–1, CO2 levels of 380 ± 5 parts per million, a block temperature of 27°C and ambient relative humidity. In each individual, three to four canopy sun leaves were randomly selected for gas exchange measurements. Values were taken after stomatal conductance reached stable values, usually no longer than 5 min after clamping the leaf on the chamber.
Manipulation of xylem delivery rates
We tested the hypothesis that a decline in Kleaf influences leaf senescence via its effect on the delivery of xylem-transported compounds by reducing leaf transpiration with a novel system of canopy humidity chambers (Fig. S2). Leaves were subject to reduced transpiration rates (but open stomata) by maintaining the chamber humidity at more than 90% during the daylight period (Fig. S3). Tomato leaves between the fourth and sixth nodes from the plant apex were selected for the high humidity treatment. The most proximal older leaf was placed in an identical control chamber at ambient humidity. Leaf chlorophyll content was monitored weekly with a SPAD meter for a maximum of 35 days or until leaves had chlorophyll contents below 25 µg cm–2, the average level we associated with the onset of leaf senescence. In tropical and temperate trees, high humidity and ambient humidity control chambers were located in partially shaded branches to avoid overheating, and next to each other to reduce microenvironmental differences. Chlorophyll was monitored in situ with a SPAD meter every week for a maximum of 33 days or until leaves senesced or fell. Diurnal measurements of PAR (LI-250, LI-COR), relative humidity (SAM990DW digital sling psychrometer, McMaster-Carr), and ambient and leaf temperature (OS543, Omega) were performed on sunny days in both high humidity and control chambers. Quantum yield and electron transport rate light curves were determined with a Mini-Pam (Walz, Eichenring, Germany) to assess the effect of the high humidity treatment on photosynthetic light reactions.
Statistical analysis
Changes in chlorophyll content with leaf age were analysed with an ANOVA and post hoc Tukey tests (SPSS, IBM, Armonk, NY, USA). Differences in physiological and anatomical properties between young and old leaves, and the effect of high humidity on leaf chlorophyll with respect to control treatments, were examined with t-tests. Calibration curves were determined using the least-squares method.
Results
Kleaf and chlorophyll content dynamics with leaf age
Tomato leaf chlorophyll content remained relatively stable until leaves were an average of 18 days old, when values began to decline (Fig. 1). The onset of leaf senescence was defined as the point at which chlorophyll declined significantly below that of young leaves. Based on the linear regression of leaf age (days) and position (LNA), we estimated that leaf senescence was triggered at an average leaf age of 27 days (ANOVA, Tukey’s post hoc test, P < 0.05; n = 5). Kleaf values tended to be highest in young leaves, where the average Kleaf was 20.9 ± 7.5 mmol H2O m–2 s–1 MPa–1. As leaves aged, Kleaf decreased to 64% of this value before the onset of senescence (ANOVA, Tukey’s post hoc test, P < 0.05; n = 5). To assess the impact of aging on leaf hydraulics independently from leaf node with respect to the apex, we also compared leaves at a fixed node from the bottom of the plant but separated by a 25-day difference in age (Fig. S4). Similarly, Kleaf declined from an average of 19.4 ± 7.0 mmol H2O m–2 s–1 MPa–1 to 9.1 ± 1.1 mmol H2O m–2 s–1 MPa–1 (t-test, P < 0.05; n = 5).
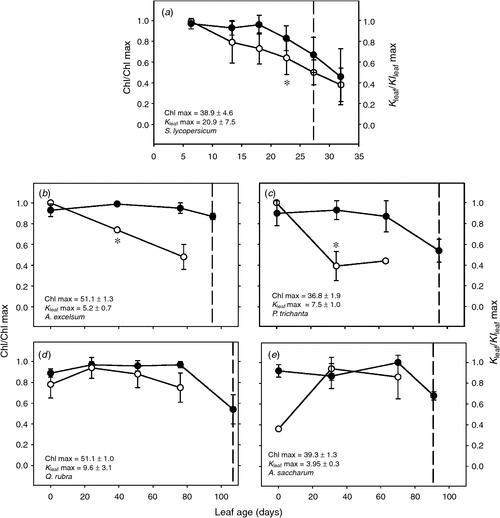
|
The selected pre-dry season A. excelsum leaf cohort exhibited a significant reduction in chlorophyll content 95 days after completing leaf expansion (ANOVA, Tukey’s post hoc test, P < 0.05; n = 3) (Fig. 1). This developmental age coincided with the median leaf longevity of 97 ± 75 days from the 1992–1994 censuses (unpubl. data by Wright et al.). The reduction in chlorophyll was significant when average values dropped to 85% of the maximum chlorophyll attained by the leaf cohort during the study period (ANOVA, Tukey’s post hoc test, P < 0.05; n = 3). At full expansion, leaves had the highest average Kleaf levels during their lifespan (5.2 ± 0.6 mmol H2O m–2 s–1 MPa–1). As the leaf cohort aged, Kleaf declined steadily reaching less than 50% of the maximum before senescence was triggered (ANOVA, Tukey’s post hoc test, P < 0.05; n = 3). Similarly, P. trichantha leaves senesced after 95 days of leaf expansion, also experiencing a reduction in Kleaf to 44% of a maximum 7.5 ± 1.0 mmol H2O m–2 s–1 MPa–1 (ANOVA, Tukey’s post hoc test, P < 0.05; n = 3). In contrast, temperate tree leaves in A. saccharum and Q. rubra did not show a reduction in hydraulic supply before senescence (Fig. 1). A significant decline in leaf chlorophyll marked the onset of senescence 91 and 107 days after expansion for A. saccharum and Q. rubra, respectively (ANOVA, Tukey’s post hoc test, P < 0.05).
Tomato hydraulic conductance dynamics in leaf lamina and rachis with age
Leaf-specific hydraulic conductance of the rachis (Krachis) dropped by 44% from young to old leaves (Fig. 2a), whereas leaflet hydraulic conductance (Kleaflet) declined by 36% (Fig. 2b) (t-test, P < 0.01 and P < 0.05 respectively; n = 6). Krachis and Kleaflet calculations involved measurements of total conductance (water flow per driving pressure, F ΔP–1) and leaf area supplied by the vascular system. Each of these components can vary independently, so we assessed their individual impact on leaf hydraulics with leaf age. Although F ΔP–1 increased in the rachis with age (Fig. 2c) (t-test, P < 0.01; n = 6), in the lamina, it remained unchanged (Fig. 2d) (t-test, P = 0.776; n = 6). In contrast, leaf area supplied by the rachis xylem (Fig. 2e) and leaflet lamina area increased from young to old leaves (Fig. 2f) (t-test, P < 0.01 and P < 0.05 respectively; n = 6). Total vein length increased as leaves aged, but not at pace with leaf expansion resulting in a continuous decline in vein density for leaves of all ages from young to old leaves (Fig. 3).
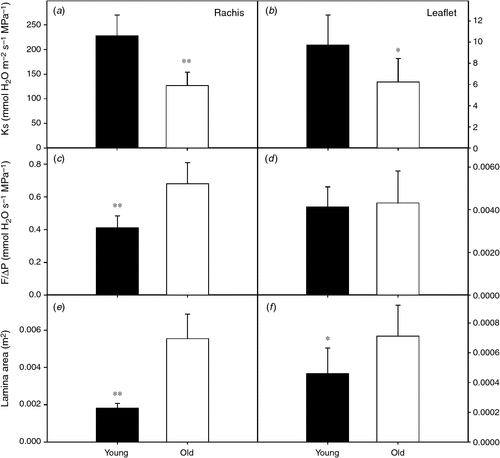
|
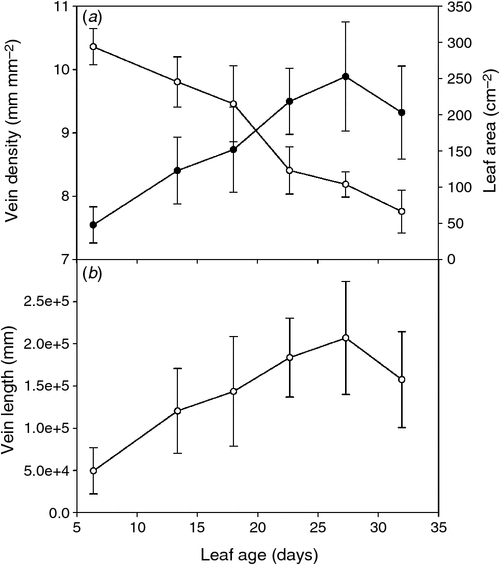
|
Changes in petiole hydraulic properties with age were not associated with modifications in xylem vessel diameter distribution (Fig. S5). Most vessels were within the 20 µm range for both young and old leaves. Despite a slightly larger number of vessels in the 40 µm category in young leaves, the differences were not significant (t-test, P = 0.097 and P = 0.438, respectively; n = 5). Only one vessel in the 60 µm range was found in old leaves but none in young leaves. Furthermore, petiole percentage loss of conductance (PLC) due to xylem embolism was comparable for leaves of both ages (t-test, P = 0.69; n = 6), averaging 17 ± 14% and 22 ± 20% for young and old leaves, respectively. However, total petiole xylem area increased with leaf age from 0.54 ± 0.15 mm2 to 1.23 ± 0.25 mm2 (t-test, P < 0.01; n = 5).
Leaf hydraulic impact on gas exchange
A significant reduction in both A and gs in tomato leaves (Fig. 4) was observed in leaves aged 22 days, after Kleaf dropped to 64% from its maximum, indicating a limitation of gas exchange by liquid phase transport. Stomatal conductance exhibited an average reduction of 59% before the onset of leaf senescence (ANOVA, Tukey’s post hoc test, P < 0.05; n = 5). Despite reductions in Kleaf in the tropical trees A. excelsum and P. trichantha to less than half of the maximum Kleaf, there was no impact on gas exchange (Fig. 4). The slightly lower assimilation rates as leaves aged in A. excelsum were associated with changes in stomatal aperture. After leaf expansion, in the temperate tree A. saccharum, stomatal conductance was maintained almost unchanged; in Q. rubra, values initially increased before reaching a plateau (Fig. 4). Similarly, assimilation was relatively constant throughout the season with the exception of a slight increase in young leaves of Q. rubra.
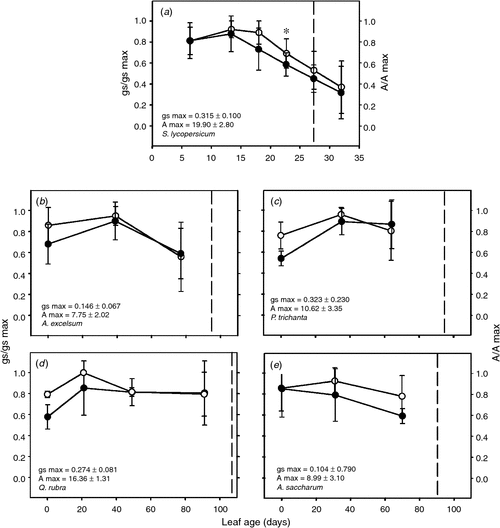
|
Effect of transpiration downregulation on leaf senescence
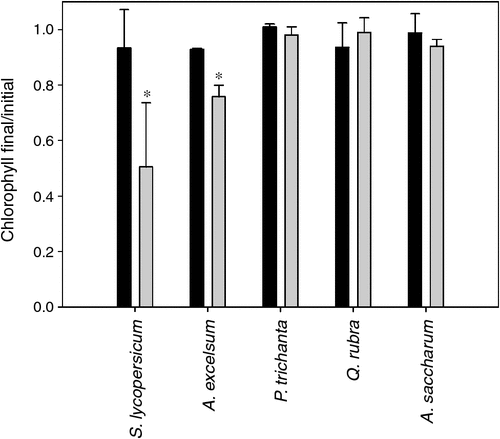
Manipulation of leaf xylem flow rates via reducing transpiration in high humidity chambers induced earlier senescence in wild-type tomato leaves (t-test, P < 0.05; n = 5; Fig. 5). Leaves under high humidity had significantly lower chlorophyll levels at the end of the treatment, despite being younger than their control counterparts. Light reactions of photosynthesis were not affected by the humidity treatment, as indicated by quantum yield and electron transport rate light curves (Figs S6, S7). Reducing xylem transport delivery with canopy humidity chambers also impacted leaf chlorophyll dynamics with age in the tropical evergreen tree A. excelsum (t-test, P < 0.05; n = 3; Fig. 5). Leaves senesced and abscised after a week of applying the treatment during the dry season in February 2009 when trees were exchanging their leaves. In the dry season of the following year (January 2010), the individual studied responded with a decline in leaf chlorophyll but senescence occurred after 33 days of applying the humidity treatment. The later manipulation experiment was initiated when the tree was not flushing new leaves in a year characterised by a late leaf exchange of A. excelsum trees around the canopy crane (personal observation, data not shown). Quantum yield and electron transport rate light curves in high humidity and control treatments exhibited overlapping patterns (Figs S6, S7). The reduction in transpiration rates in the high humidity treatments did not impact the timing of leaf senescence in the deciduous tropical tree P. trichantha and the temperate trees A. saccharum and Q. rubra (Fig. 5). Despite leaves being exposed to mean diurnal VPD values in average five times lower than the ambient for more than a month (Fig. S8), chlorophyll content remained at similar levels in the canopy humidity and control chambers for these tree species. Quantum yield and electron transport rate light curves were comparable in leaves of both treatments in P. trichantha and A. saccharum but not in Q. rubra, indicating a slight reduction of photosynthesis light reactions in canopy humidity chambers (Figs S6, S7). Diurnal patterns of leaf temperatures, air temperature and PAR were similar across treatments (Fig. S8). As expected, VPD values were lower in the humidity treatment.
|
Discussion
Age-related changes in leaf anatomy leads to Kleaf downregulation before the onset of tomato leaf senescence
Both Krachis and Kleaflet contributed to the decline in Kleaf before the onset of senescence in tomato leaves. Krachis and Kleaflet experienced an equivalent reduction in conductance of more than half of their initial capacity at early developmental stages. However, the hydraulic conductance of the rachis was more than 20 times higher than the lamina in both leaf age categories. Thus the conductance of the lamina may have constituted a leaf hydraulic bottleneck that influenced, to a larger extent, the age-dependent decline in Kleaf. The low hydraulic conductance of extraxylary pathways has been reported in angiosperm leaves (Cochard et al. 2004; Gascó et al. 2004). However, other studies have proposed a greater amount of conductance to water flow in the mesophyll relative to the xylem of leaf blades (Sack et al. 2002) and the minor contribution of rachis conductance to total fern frond water transport capacity (Lo Gullo et al. 2010), underscoring the need for a better understanding of hydraulic conductance partitioning within compound leaves.
In glasshouse-grown tomatoes, age-related changes due to endogenous factors rather than environmental stressors are the most parsimonious explanation of impaired leaf hydraulic function. Modifications in the hydraulic architecture of the lamina and rachis as leaves aged were associated with the observed decline in Kleaf. The maintenance of similar flow rates per driving pressure in the leaf lamina indicated that the total leaflet conductance across developmental stages remained unchanged. However, parallel reductions in leaf vein density with increases in leaf area with age suggest that the leaflet vascular system does not develop an adequate hydraulic supply to comply with lamina expansion. These changes in leaf anatomy with age echo findings that report that lower vein density in basal relative to apical leaves is associated with a decline in Kleaf (Nardini et al. 2008). Whether the relative hydraulic conductance of xylary to extraxylary pathways changes with leaf expansion in tomato remains unknown. Nardini et al. (2010) reported a concurrent trend of decrease in extravascular and vascular Kleaf with leaf area expansion accompanied by a shift from extravascular-dominated conductance at early stages of expansion to higher vascular conductance in fully expanded leaves. Ontogenetic changes in the hydraulic conductance of the leaf mesophyll have been associated with an increase in palisade and spongy cell density, which forces water to cross a higher frequency of cell membranes (Aasamaa et al. 2005), in which the majority of the resistance to leaf water flux may occur (Ye et al. 2008).
The reduction in Krachis could not be attributed to changes of xylem flow rates per driving pressure, as the total rachis conductance increased with age, indicating a higher hydraulic supply. Neither petiole vessel size distributions nor PLC were associated with changes in Krachis across leaf age categories, but PLC values reflected considerable amounts of embolism at both leaf developmental stages. Alternatively, vascular bundle radial hydraulic conductance, instead of conduit diameter, could have determined rachis hydraulics, as in ferns (Lo Gullo et al. 2010), but it remains to be tested if pit membrane permeability in angiosperm leaves is as great a contributor to leaf xylem resistance as it is in stems (Sperry et al. 2005). Our results contrast with those regarding Populus tremula Michx., in which midrib conduit size, but not cross-sectional area, was the driver in Kleaf changes during leaf ontogeny (Aasamaa et al. 2005). Only larger xylem conductive area in old tomato leaves could explain the increase in total rachis conductance. However, increased xylem hydraulic supply in the rachis in old leaves was not sufficient to compensate for leaf area expansion, leading to a net Krachis decline.
Leaf vascular supply influences the onset of age-dependent leaf senescence in tomato
Many studies have recognised that leaf senescence is an age-dependent process (Lim et al. 2007). We propose that in tomato, Kleaf can act as an age-dependent signal determining the timing of leaf senescence by limiting gas exchange and thus xylem-transported compound flow into leaves. The early induction of leaf senescence by transpiration downregulation in canopy humidity chambers, independent of light levels, suggests a role of xylem-transported compounds in determining leaf senescence in tomato. The age-dependent decline in Kleaf of tomato plants associated with a reduction in gas exchange also indicates a limitation to xylem compound delivery. Consistent with this view, previous studies have shown that the delivery of xylem-transported compounds such as cytokinins are dependent on transpiration rates in tobacco and A. thaliana (Boonman et al. 2007; Boonman et al. 2009). Similarly, rootstock-mediated manipulations of xylem-transported cytokinins in tomato plants point to their role in delaying leaf senescence under high salinity conditions (Ghanem et al. 2008; Albacete et al. 2009).
Kleaf dowregulation with age was driven by a reduction in leaf vein density associated with leaf lamina expansion. As leaves age, neither the xylary pathways nor the lamina vascular system develop the hydraulic supply required by a larger leaf conductive area. Hydraulic limitations in the leaf lamina have been shown to play a key role in determining leaf size and shape (Zwieniecki et al. 2004; Carins Murphy et al. 2012; Zhang et al. 2012). Similarly, as Kleaf began to limit gas exchange in tomato leaves, lamina expansion ceased. Constraining Kleaf dynamics to leaf expansion provides a simple but consistent age-dependent mechanism to control the timing of leaf senescence via regulation of the flow of xylem-transported compounds. An adaptive advantage of the proposed mechanism is that it may allow plants to recognise the developmental age of leaves for nutrient translocation during new leaf growth or plant reproduction.
Kleaf does not affect leaf senescence in tropical A. excelsum and P. trichantha and temperate A. saccharum and Q. rubra trees
Leaf vascular influence on senescence appeared consistent with Kleaf reduction before a decline in chlorophyll content in A. excelsum and A. trichanta. However, there was no gas exchange downregulation with leaf age, indicating that Kleaf does not limit the flow of xylem-transported compounds. Tropical tree species vary in the degree of gas exchange response to Kleaf decline. Some show a strong correlation between Kleaf and their gas exchange recovery during rehydration; in others, stomatal conductance is insensitive to artificially depressed Kleaf (Brodribb and Holbrook 2007; Blackman et al. 2009; Brodribb and Cochard 2009). The insensitive species have been linked to a linear decline in Kleaf with leaf water potential, whereas more sensitive species exhibit a sigmoidal curve (Brodribb and Holbrook 2007). A. excelsum’s and P. trichantha’s Kleaf average values below 7.5 mmol H2O m–2 s–1 MPa–1 fall within the low range reported for tropical woody angiosperm species (Brodribb et al. 2005; Sack and Holbrook 2006), where there should be a strong correlation between Kleaf and maximum carbon assimilation (Brodribb et al. 2005). However, we recorded only a slight or no decrease in assimilation and stomatal conductance.
In the tropical deciduous tree P. trichantha, reducing leaf transpiration in high humidity chambers had no impact on chlorophyll dynamics; however, in evergreen A. excelsum trees, early senescence was triggered only during leaf exchange events in the dry season. Reducing leaf xylem transport delivery is, therefore, not a sufficient condition for the induction of senescence. Future studies should examine the hypothesis of a nitrogen sink requirement for the occurrence of age-dependent leaf senescence in A. excelsum. It is well known that the leaves of herbaceous plants grown at high nitrogen tend to senesce later (Ono et al. 2001). Delaying leaf senescence using nitrogen fertilisation has been reported in the temperate tree Populus trichocarpa Tor. & Gray (Sigurdsson 2001). However, how xylem-transported nitrogen affects tropical leaf phenology is not well understood. According to this framework, the leaf senescence program could be activated in evergreen tropical trees when sink organs like young leaves, flowers or fruits initiate their development. Evidence supporting this hypothesis comes from the seasonal leaf dynamics and reproductive phenology censuses from 1992 to 1994, recording leaf mortality increases together with the induction of new leaf and reproductive growth in A. excelsum (unpubl. data by Wright et al.). The physiological mechanisms regulating leaf senescence in tropical trees with diverse phenological strategies remain to be explored (Giraldo and Holbrook 2011).
Our results indicate that the mechanisms regulating leaf senescence of the temperate tree species A. saccharum and Q. rubra do not involve changes in leaf vascular supply. Average Kleaf values were constant before senescence and were comparable to those reported with the vacuum technique (Lo Gullo et al. 2005) but lower than measured with evaporative flux method (Sack et al. 2002). The divergences could be a consequence of this study’s working range of more negative water potentials and higher flow rates. Our findings also contrast with leaf hydraulic dynamics studies of Q. rubra trees planted in the Mediterranean region in which a 70% drop in seasonal Kleaf occurred before any visible changes in leaf colouring (Lo Gullo et al. 2005), suggesting a plastic response of leaf hydraulic properties to seasonal variations in environmental conditions. Furthermore, reduction of leaf transpiration rates in canopy humidity chambers lasting more than a month did not trigger early leaf senescence. Together, our results indicate that xylem delivery does not impact the timing of leaf senescence in these temperate tree species.
Nevertheless, we cannot rule out the proposed hydraulic mechanism for regulating leaf senescence via xylem compound delivery for other temperate tree species. Salleo et al. (2002) identified a reduction in Kleaf during the photosynthetic season preceding any visible changes in Castanea sativa L. leaf colouring, leading the authors to hypothesise that widespread xylem blockage in both the stem and leaves due to the formation of embolism, followed by tyloses growing into the conduits, could initiate leaf shedding in this temperate tree species. It has also been proposed that ring-porous temperate hardwood species that lose early wood vessels every year (Zimmermann 1983) tend to senesce earlier in the autumn than their diffuse-porous counterparts due to summer drought-induced loss in xylem function (Wang et al. 1992). Seasonal reductions in stem and leaf hydraulic supply have been linked to strong coordination with stomatal conductance (Lo Gullo et al. 2005) but the impact of variations in temperate trees’ hydraulic resistance on the delivery of xylem-transported compounds via gas exchange limitation remains poorly understood.
Acknowledgements
We thank Joseph Wright and Klaus Winter for helping to provide laboratory space and equipment at Smithsonian Tropical Research Institute. Mirna Samaniego contributed with leaf censuses and logistical support at the Smithsonian Tropical Research Institute canopy crane. Senior Harvard Research Experience for Undergraduates (REU) students Lee Dietterich and James Onstad assisted with field data collection at Harvard Forest. Maciej Zwieniecki and Fulton Rockwell made insightful comments on experimental design. Jessica Savage and Matthew Gilbert provided valuable reviews of the manuscript. We gratefully acknowledge funds awarded to JPG for research and travel expenses provided by the Smithsonian Predoctoral Fellowship Program. This article was partially elaborated while the leading author was a Giorgio Ruffolo Fellow in the Sustainability Science Program at Harvard University with support from Italy’s Ministry for Environment, Land and Sea. We also gratefully acknowledge funding from the Andrew W Mellon Foundation awarded to NMH.
References
Aasamaa K, Niinemets U, Sober A (2005) Leaf hydraulic conductance in relation to anatomical and functional traits during Populus tremula leaf ontogeny. Tree Physiology 25, 1409–1418.| Leaf hydraulic conductance in relation to anatomical and functional traits during Populus tremula leaf ontogeny.Crossref | GoogleScholarGoogle Scholar | 16105808PubMed |
Albacete A, Martínez-Andújar C, Ghanem ME, Acosta M, Sánchez-Bravo J, Asins MJ, Cuartero J, Lutts S, Dodd IC, Pérez-Alfocea F (2009) Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato. Plant, Cell & Environment 32, 928–938.
| Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosl2jsb0%3D&md5=8d8968b04d1c348e8d823c7638e13300CAS |
Berlyn GP, Miksche JP (1976) Botanical microtechnique and cytochemistry. (Iowa State University Press: Ames, Iowa)
Blackman CJ, Brodribb TJ, Jordan GJ (2009) Leaf hydraulics and drought stress: response, recovery and survivorship in four woody temperate plant species. Plant, Cell & Environment 32, 1584–1595.
| Leaf hydraulics and drought stress: response, recovery and survivorship in four woody temperate plant species.Crossref | GoogleScholarGoogle Scholar |
Boonman A, Prinsen E, Gilmer F, Schurr U, Peeters AJM, Voesenek L, Pons TL (2007) Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients. Plant Physiology 143, 1841–1852.
| Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksFWjtbY%3D&md5=b5d3f00c4df91e82c6ff9d2f2dfc19feCAS | 17277095PubMed |
Boonman A, Prinsen E, Voesenek L, Pons TL (2009) Redundant roles of photoreceptors and cytokinins in regulating photosynthetic acclimation to canopy density. Journal of Experimental Botany 60, 1179–1190.
| Redundant roles of photoreceptors and cytokinins in regulating photosynthetic acclimation to canopy density.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjsFShs7s%3D&md5=76e5e4e3bc94586ddb48ae2a4ffafd70CAS | 19240103PubMed |
Brodribb TJ, Cochard H (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology 149, 575–584.
| Hydraulic failure defines the recovery and point of death in water-stressed conifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt1Wqtb4%3D&md5=1263655147532700e5bc4b8701254f2dCAS | 19011001PubMed |
Brodribb TJ, Holbrook NM (2003a) Changes in leaf hydraulic conductance during leaf shedding in seasonally dry tropical forest. New Phytologist 158, 295–303.
| Changes in leaf hydraulic conductance during leaf shedding in seasonally dry tropical forest.Crossref | GoogleScholarGoogle Scholar |
Brodribb TJ, Holbrook NM (2003b) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology 132, 2166–2173.
| Stomatal closure during leaf dehydration, correlation with other leaf physiological traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmsVantbc%3D&md5=5e37abae6f351712a8923a2cc0de35c9CAS | 12913171PubMed |
Brodribb TJ, Holbrook NM (2006) Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant, Cell & Environment 29, 2205–2215.
| Declining hydraulic efficiency as transpiring leaves desiccate: two types of response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVaitA%3D%3D&md5=3b48a1de64db28b3db03cef1f723c0dfCAS |
Brodribb TJ, Holbrook NM (2007) Forced depression of leaf hydraulic conductance in situ: effects on the leaf gas exchange of forest trees. Functional Ecology 21, 705–712.
| Forced depression of leaf hydraulic conductance in situ: effects on the leaf gas exchange of forest trees.Crossref | GoogleScholarGoogle Scholar |
Brodribb T, Holbrook N, Zwieniecki M, Palma B (2005) Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. New Phytologist 165, 839–846.
| Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima.Crossref | GoogleScholarGoogle Scholar | 15720695PubMed |
Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898.
| Leaf maximum photosynthetic rate and venation are linked by hydraulics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVOgs7s%3D&md5=0f0bcc31dbcd41cfef4c9194f51ea13dCAS | 17556506PubMed |
Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D (2003) The molecular analysis of leaf senescence – a genomics approach. Plant Biotechnology Journal 1, 3–22.
| The molecular analysis of leaf senescence – a genomics approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptV2ktrs%3D&md5=914208c7a9972399529c5b1cf380e554CAS | 17147676PubMed |
Carins Murphy MR, Jordan GJ, Brodribb TJ (2012) Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant, Cell & Environment 35, 1407–1418.
| Differential leaf expansion can enable hydraulic acclimation to sun and shade.Crossref | GoogleScholarGoogle Scholar |
Cochard H, Nardini A, Coll L (2004) Hydraulic architecture of leaf blades: where is the main resistance? Plant, Cell & Environment 27, 1257–1267.
| Hydraulic architecture of leaf blades: where is the main resistance?Crossref | GoogleScholarGoogle Scholar |
Estrella N, Menzel A (2006) Responses of leaf colouring in four deciduous tree species to climate and weather in Germany. Climate Research 32, 253–267.
| Responses of leaf colouring in four deciduous tree species to climate and weather in Germany.Crossref | GoogleScholarGoogle Scholar |
Gan SS, Amasino RM (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270, 1986–1988.
| Inhibition of leaf senescence by autoregulated production of cytokinin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xlt12q&md5=9ee325b03921b5ebf5a01004bde3024bCAS |
Gan SS, Amasino RM (1996) Cytokinins in plant senescence: from spray and pray to clone and play. BioEssays 18, 557–565.
| Cytokinins in plant senescence: from spray and pray to clone and play.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XkvVWktLc%3D&md5=12aad31f9f8ccf7d1b47458dcf888b2bCAS |
Gascó A, Nardini A, Salleo S (2004) Resistance to water flow through leaves of Coffea arabica is dominated by extra-vascular tissues. Functional Plant Biology 31, 1161–1168.
| Resistance to water flow through leaves of Coffea arabica is dominated by extra-vascular tissues.Crossref | GoogleScholarGoogle Scholar |
Ghanem ME, Albacete A, Martinez-Andujar C, Acosta M, Romero-Aranda R, Dodd IC, Lutts S, Perez-Alfocea F (2008) Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). Journal of Experimental Botany 59, 3039–3050.
| Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpslalsb4%3D&md5=3e3f157ff2706135d5a71fcb168e321fCAS | 18573798PubMed |
Giraldo JP, Holbrook NM (2011) Physiological mechanisms underlying the seasonality of leaf senescence and renewal in seasonally dry tropical forests trees. In ‘Seasonally dry tropical forests: ecology and conservation’. (Eds R Dirzo, H Young, H Mooney, G Ceballos) pp. 129–140. (Island Press: Washington DC)
Guo YF, Gan SS (2005) Leaf senescence: signals, execution, and regulation. In ‘Current topics in developmental biology’. (Eds G Schatten) Vol. 71, pp. 83–112. (Elsevier Academic Press: San Diego)
Hensel LL, Grbic V, Baumgarten DA, Bleecker AB (1993) Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in Arabidopsis. The Plant Cell 5, 553–564.
Himelblau E, Amasino RM (2001) Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. Journal of Plant Physiology 158, 1317–1323.
| Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXosl2mu7k%3D&md5=1f74e675c6e54d2057df27e863603443CAS |
Hörtensteiner S (2006) Chlorophyll degradation during senescence. Annual Review of Plant Biology 57, 55–77.
| Chlorophyll degradation during senescence.Crossref | GoogleScholarGoogle Scholar | 16669755PubMed |
Kaldenhoff R, Fischer M (2006) Aquaporins in plants. Acta Physiologica (Oxford, England) 187, 169–176.
| Aquaporins in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlvVCgurs%3D&md5=58b3ad214d324c26cf53fd78dfd8198bCAS |
Kaldenhoff R, Ribas-Carbo M, Flexas J, Lovisolo C, Heckwolf M, Uehlein N (2008) Aquaporins and plant water balance. Plant, Cell & Environment 31, 658–666.
| Aquaporins and plant water balance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvFehur8%3D&md5=e5452be32662aba1cff66727d201fa34CAS |
Lee DW, O’Keefe J, Holbrook NM, Feild TS (2003) Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecological Research 18, 677–694.
| Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhsVClt70%3D&md5=245a0897b56a3bf510320f08d1a85f1eCAS |
Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annual Review of Plant Biology 58, 115–136.
| Leaf senescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnsVahs70%3D&md5=af29ba153b44fac5167ae4448786c096CAS | 17177638PubMed |
Lo Gullo MA, Noval LC, Salleo S, Nardini A (2004) Hydraulic architecture of plants of Helianthus annuus L. cv. Margot: evidence for plant segmentation in herbs. Journal of Experimental Botany 55, 1549–1556.
| Hydraulic architecture of plants of Helianthus annuus L. cv. Margot: evidence for plant segmentation in herbs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltlOqu7w%3D&md5=1fca5c17333a2a28048449d7b53c96faCAS | 15181104PubMed |
Lo Gullo MA, Nardini A, Trifilo P, Salleo S (2005) Diurnal and seasonal variations in leaf hydraulic conductance in evergreen and deciduous trees. Tree Physiology 25, 505–512.
| Diurnal and seasonal variations in leaf hydraulic conductance in evergreen and deciduous trees.Crossref | GoogleScholarGoogle Scholar | 15687099PubMed |
Lo Gullo MA, Raimondo F, Crisafulli A, Salleo S, Nardini A (2010) Leaf hydraulic architecture and water relations of three ferns from contrasting light habitats. Functional Plant Biology 37, 566–574.
| Leaf hydraulic architecture and water relations of three ferns from contrasting light habitats.Crossref | GoogleScholarGoogle Scholar |
McLaughlin SB, Wimmer R (1999) Calcium physiology and terrestrial ecosystem processes. New Phytologist 142, 373–417.
| Calcium physiology and terrestrial ecosystem processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlsFGrs7c%3D&md5=2ab8ff8025642a5697b555557ddba9e1CAS |
Meinzer FC (2002) Co-ordination of vapour and liquid phase water transport properties in plants. Plant, Cell & Environment 25, 265–274.
| Co-ordination of vapour and liquid phase water transport properties in plants.Crossref | GoogleScholarGoogle Scholar |
Miller A, Schlagnhaufer C, Spalding M, Rodermel S (2000) Carbohydrate regulation of leaf development: prolongation of leaf senescence in Rubisco antisense mutants of tobacco. Photosynthesis Research 63, 1–8.
| Carbohydrate regulation of leaf development: prolongation of leaf senescence in Rubisco antisense mutants of tobacco.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlsVymtLY%3D&md5=7e25588100aba0781079ea036b85f3d3CAS | 16252160PubMed |
Munné-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Functional Plant Biology 31, 203–216.
| Die and let live: leaf senescence contributes to plant survival under drought stress.Crossref | GoogleScholarGoogle Scholar |
Nardini A, Salleo S (2005) Water stress-induced modifications of leaf hydraulic architecture in sunflower: co-ordination with gas exchange. Journal of Experimental Botany 56, 3093–3101.
| Water stress-induced modifications of leaf hydraulic architecture in sunflower: co-ordination with gas exchange.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1GlsbbO&md5=17590d968758405fb1107ab5defa1b22CAS | 16246857PubMed |
Nardini A, Gortan E, Ramani M, Salleo S (2008) Heterogeneity of gas exchange rates over the leaf surface in tobacco: an effect of hydraulic architecture? Plant, Cell & Environment 31, 804–812.
| Heterogeneity of gas exchange rates over the leaf surface in tobacco: an effect of hydraulic architecture?Crossref | GoogleScholarGoogle Scholar |
Nardini A, Raimondo F, Lo Gullo MA, Salleo S (2010) Leafminers help us understand leaf hydraulic design. Plant, Cell & Environment 33, 1091–1100.
Ono K, Watanabe A (1997) Levels of endogenous sugars, transcripts of rbcS and rbcL, and of Rubisco protein in senescing sunflower leaves. Plant & Cell Physiology 38, 1032–1038.
| Levels of endogenous sugars, transcripts of rbcS and rbcL, and of Rubisco protein in senescing sunflower leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmtlyltrc%3D&md5=0f5a2bd758081203e5f15f44b3b42fdeCAS |
Ono K, Nishi Y, Watanabe A, Terashima I (2001) Possible mechanisms of adaptive leaf senescence. Plant Biology 3, 234–243.
| Possible mechanisms of adaptive leaf senescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvFGhsb4%3D&md5=91c21dced2924155b110d6aa49753ea5CAS |
Pourtau N, Mares M, Purdy S, Quentin N, Ruel A, Wingler A (2004) Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta 219, 765–772.
| Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnt1GlurY%3D&md5=a892b47b4d46986d3d6319a62ab6007dCAS | 15118859PubMed |
Richmond AE, Lang A (1957) Effect of kinetin on protein content and survival of detached Xanthium leaves. Science 125, 650–651.
| Effect of kinetin on protein content and survival of detached Xanthium leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG2sXkslOktA%3D%3D&md5=c8c026c8ec96992bf36d6dfdc9418095CAS |
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology 57, 675–709.
| Sugar sensing and signaling in plants: conserved and novel mechanisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosVKht7k%3D&md5=51c252f22fd5eba6aecaf76c949f2ee7CAS | 16669778PubMed |
Sack L, Holbrook NM (2006) Leaf hydraulics. Annual Review of Plant Biology 57, 361–381.
| Leaf hydraulics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosVKhtrs%3D&md5=3f502b84989933bacc5535c1045bdb19CAS | 16669766PubMed |
Sack L, Melcher PJ, Zwieniecki MA, Holbrook NM (2002) The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. Journal of Experimental Botany 53, 2177–2184.
| The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xpt1KgtLg%3D&md5=83c383fd6a58330126fe336037d6e958CAS | 12379784PubMed |
Salleo S, Nardini A, Lo Gullo MA, Ghirardelli LA (2002) Changes in stem and leaf hydraulics preceding leaf shedding in Castanea sativa L. Biologia Plantarum 45, 227–234.
| Changes in stem and leaf hydraulics preceding leaf shedding in Castanea sativa L.Crossref | GoogleScholarGoogle Scholar |
Scoffoni C, Sack L, PrometheusWiki contributors (2010) ‘Quantifying leaf vein traits.’ PrometheusWiki. Available at: http://www.publish.csiro.au/prometheuswiki/tiki-pagehistory.php?page=Quantifying leaf vein traits&preview=14
Sigurdsson BD (2001) Elevated [CO2] and nutrient status modified leaf phenology and growth rhythm of young Populus trichocarpa trees in a 3-year field study. Trees – Structure and Function 15, 403–413.
| Elevated [CO2] and nutrient status modified leaf phenology and growth rhythm of young Populus trichocarpa trees in a 3-year field study.Crossref | GoogleScholarGoogle Scholar |
Sperry JS, Perry AH, Sullivan JEM (1991) Pit membrane degradation and air-embolism formation in aging xylem vessels of Populus tremuloides Michx. Journal of Experimental Botany 42, 1399–1406.
| Pit membrane degradation and air-embolism formation in aging xylem vessels of Populus tremuloides Michx.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XlsFKksA%3D%3D&md5=a1cba4c79af8af9e3734f0a464d40a58CAS |
Sperry JS, Hacke UG, Wheeler JK (2005) Comparative analysis of end wall resistivity in xylem conduits. Plant, Cell & Environment 28, 456–465.
| Comparative analysis of end wall resistivity in xylem conduits.Crossref | GoogleScholarGoogle Scholar |
Swartzberg D, Dai N, Gan S, Amasino R, Granot D (2006) Effects of cytokinin production under two SAG promoters on senescence and development of tomato plants. Plant Biology 8, 579–586.
| Effects of cytokinin production under two SAG promoters on senescence and development of tomato plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFSktbjF&md5=87849b452102a878ead6093ae39dbc34CAS | 16883480PubMed |
Thomas H, Ougham H, Canter P, Donnison I (2002) What stay-green mutants tell us about nitrogen remobilization in leaf senescence. Journal of Experimental Botany 53, 801–808.
| What stay-green mutants tell us about nitrogen remobilization in leaf senescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivFSntLo%3D&md5=643d76fd286b1ad0a0c4de16c32d3530CAS | 11912223PubMed |
van Doorn WG (2008) Is the onset of senescence in leaf cells of intact plants due to low or high sugar levels? Journal of Experimental Botany 59, 1963–1972.
| Is the onset of senescence in leaf cells of intact plants due to low or high sugar levels?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmvFWku7g%3D&md5=817628c9df842dfc432d65ce06e45ea3CAS | 18453532PubMed |
Wang J, Ives NE, Lechowicz MJ (1992) The relation of foliar phenology to xylem embolism in trees. Functional Ecology 6, 469–475.
| The relation of foliar phenology to xylem embolism in trees.Crossref | GoogleScholarGoogle Scholar |
Wingler A, Mares M, Pourtau N (2004) Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence. New Phytologist 161, 781–789.
| Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence.Crossref | GoogleScholarGoogle Scholar |
Woo HR, Goh CH, Park JH, de la Serve BT, Kim JH, Park YI, Nam HG (2002) Extended leaf longevity in the ore4–1 mutant of Arabidopsis with a reduced expression of a plastid ribosomal protein gene. The Plant Journal 31, 331–340.
| Extended leaf longevity in the ore4–1 mutant of Arabidopsis with a reduced expression of a plastid ribosomal protein gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XntFelsb4%3D&md5=2775e68cdfbac239d42a5c70e83fc36fCAS | 12164812PubMed |
Wright SJ (1991) Seasonal drought and the phenology of understory shrubs in a tropical moist forest. Ecology 72, 1643–1657.
| Seasonal drought and the phenology of understory shrubs in a tropical moist forest.Crossref | GoogleScholarGoogle Scholar |
Wright SJ, Cornejo FH (1990) Seasonal drought and leaf fall in a tropical forest. Ecology 71, 1165–1175.
| Seasonal drought and leaf fall in a tropical forest.Crossref | GoogleScholarGoogle Scholar |
Wright SJ, Vanschaik CP (1994) Light and the phenology of tropical trees. American Naturalist 143, 192–199.
| Light and the phenology of tropical trees.Crossref | GoogleScholarGoogle Scholar |
Ye Q, Holbrook NM, Zwieniecki MA (2008) Cell-to-cell pathway dominates xylem-epidermis hydraulic connection in Tradescantia fluminensis (Vell. Conc.) leaves. Planta 227, 1311–1319.
| Cell-to-cell pathway dominates xylem-epidermis hydraulic connection in Tradescantia fluminensis (Vell. Conc.) leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXks1Ght78%3D&md5=c520177fddea64ffa7eeeae276dadf77CAS | 18273638PubMed |
Zhang YX, Equiza MA, Zheng QS, Tyree MT (2012) Factors controlling plasticity of leaf morphology in Robinia pseudoacacia L. II: the impact of water stress on leaf morphology of seedlings grown in a controlled environment chamber. Annals of Forest Science 69, 39–47.
| Factors controlling plasticity of leaf morphology in Robinia pseudoacacia L. II: the impact of water stress on leaf morphology of seedlings grown in a controlled environment chamber.Crossref | GoogleScholarGoogle Scholar |
Zimmermann MH (1983) ‘Xylem structure and the ascent of sap.’ (Springer-Verlag: Berlin)
Zwieniecki MA, Boyce CK, Holbrook NM (2004) Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant, Cell & Environment 27, 357–365.
| Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves.Crossref | GoogleScholarGoogle Scholar |


