Modelling the effect of plant water use traits on yield and stay-green expression in sorghum
Jana Kholová A D , Tharanya Murugesan A , Sivasakthi Kaliamoorthy A , Srikanth Malayee A , Rekha Baddam A , Graeme L. Hammer B , Greg McLean C , Santosh Deshpande A , C. Thomas Hash A , Peter Q. Craufurd A and Vincent Vadez AA International Crops Research Institute for the Semi-Arid Tropics, Patancheru, Andhra Pradesh 502 324, India.
B The University of Queensland, Queensland Alliance for Agriculture and Food Innovation, Brisbane, Qld 4072, Australia.
C Agri-Science Queensland, Department of Agriculture, Forestry and Fisheries, Toowoomba, Qld 4350, Australia.
D Corresponding author. Email: j.kholova@cgiar.org
This paper originates from a presentation at the Interdrought IV Conference, Perth, Australia, 2–6 September 2013.
Functional Plant Biology 41(11) 1019-1034 https://doi.org/10.1071/FP13355
Submitted: 13 December 2013 Accepted: 23 May 2014 Published: 25 July 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Post-rainy sorghum (Sorghum bicolor (L.) Moench) production underpins the livelihood of millions in the semiarid tropics, where the crop is affected by drought. Drought scenarios have been classified and quantified using crop simulation. In this report, variation in traits that hypothetically contribute to drought adaptation (plant growth dynamics, canopy and root water conducting capacity, drought stress responses) were virtually introgressed into the most common post-rainy sorghum genotype, and the influence of these traits on plant growth, development, and grain and stover yield were simulated across different scenarios. Limited transpiration rates under high vapour pressure deficit had the highest positive effect on production, especially combined with enhanced water extraction capacity at the root level. Variability in leaf development (smaller canopy size, later plant vigour or increased leaf appearance rate) also increased grain yield under severe drought, although it caused a stover yield trade-off under milder stress. Although the leaf development response to soil drying varied, this trait had only a modest benefit on crop production across all stress scenarios. Closer dissection of the model outputs showed that under water limitation, grain yield was largely determined by the amount of water availability after anthesis, and this relationship became closer with stress severity. All traits investigated increased water availability after anthesis and caused a delay in leaf senescence and led to a ‘stay-green’ phenotype. In conclusion, we showed that breeding success remained highly probabilistic; maximum resilience and economic benefits depended on drought frequency. Maximum potential could be explored by specific combinations of traits.
Additional keywords: APSIM, drought stress, Sorghum bicolor (L.) Moench, trait modelling.
Introduction
Post-rainy (rabi) sorghum (Sorghum bicolor (L.) Moench) production is the staple source of livelihood for millions of food-insecure households in semiarid tropical regions of the Indian subcontinent (Murty et al. 2007) but its production is often limited by water availability, resulting in a high risk of crop failure (Kholová et al. 2013). Sorghum is also important for the subsistence economy of many households across the semiarid tropics of Africa, where it also faces water stress. Therefore, finding innovative and faster ways for improving sorghum productivity and resilience under water-limited conditions is a must.
The ‘stay-green’ phenotype has been described as the best characterised trait contributing to drought adaptation in sorghum (e.g. Borrell and Hammer 2000; Borrell et al. 2001; Jordan et al. 2003; Harris et al. 2007; Kassahun et al. 2010). The potential of stay-green technology could be fully explored for crop improvement only if the physiological mechanisms underlying this phenomenon were properly understood. Aside from ‘cosmetic’ stay-green (retention of nonfunctional chlorophyll; see reviews (e.g. Thomas and Howarth 2000; Cha et al. 2002)), there are basically two parallel streams of hypotheses explaining the maintenance of green leaves under water stress, one of which deals with the enhanced use of available N (Rajcan and Tollenaar 1999; Borrell and Hammer 2000; Borrell et al. 2001; Bertheloot et al. 2008; van Oosterom et al. 2010b) and another one favouring an improved plant water use status (van Oosterom et al. 2010b; Vadez et al. 2011, 2014). Several quantitative trait loci (QTLs) contributing to stay-green phenotype expression under drought (Stages 1–4, Stages A and B) have been validated across different research groups (Tuinstra et al. 1996, 1997, 1998; Crasta et al. 1999; Subudhi et al. 2000; Tao et al. 2000; Xu et al. 2000; Kebede et al. 2001; Sanchez et al. 2002; Haussmann et al. 2002; Hash et al. 2003; Harris et al. 2007). Introgressing stay-green QTLs into two senescent parental lines (R16, S35) from the stay-green donor B35 produced lines showing differences in several traits related to the plant water budget (e.g. transpiration efficiency (TE), water extraction, leaf area) (Vadez et al. 2011). Here, we test the effect of several possible mechanisms affecting the plant water budget on stay-green expression, and grain and stover yield.
Breeding for improved varieties for water-limited environments has been slow, especially in developing countries, mostly because of the highly unpredictable drought environments. To tackle the season-to-season environmental variation, we have already classified and quantified five different stress scenarios across the rabi sorghum tract using the sorghum crop model in the APSIM software platform (Hammer et al. 2010; Keating et al. 2003). Environment types ranged from very severe (an average grain yield of ~100 kg ha–1) to no stress conditions (an average grain yield of ~1500 kg ha–1) and divided the rabi sorghum tract into four zones with similar environmental conditions (Kholová et al. 2013). This follows similar efforts in sorghum in Australia ((Chapman et al. 2000a, 2000b, 2000c), wheat (Triticum aestivum L.) (Chenu et al. 2011, 2013), maize (Zea mays L.) (Chauhan et al. 2013) or chickpea (Cicer arietinum L.) (Chauhan et al. 2008)). For the rabi sorghum region, which is the object of the current study, we also found that for the recommended management practices, the crop suffered severe water limitations causing substantial yield losses (typically more than half of the yield is lost during severe droughts) in around one-third of the seasons in the core production zone. Given these large variations in stress conditions, it is very likely that potential beneficial traits may not have similar effects in all scenarios, as noted by van Oosterom et al. (2001), Bidinger et al. (2007), Chapman et al. (2000a, 2000b, 2000c) and Tardieu (2012). Therefore, we have tested the effect of several traits involved in the plant water budget on grain and stover yield across the different stress scenarios that were previously identified.
Hence, the main aims of this study were to: (i) assess the genetic variation in the mechanisms and traits putatively related to the plant water budget in a series of introgression lines (ILs) developed into two genetic backgrounds (S35, R16); and (ii) use these ranges of genetic variation to model their putative effect on grain and stover yield across five different stress scenarios, using the most common rabi sorghum genotype (M35–1) as an in silico recipient of these traits; and (iii) assess the value of several mechanisms and traits in eventually conferring a stay-green phenotype and for crop improvement programs.
Materials and methods
Plant material
Two senescent recurrent parental lines (R16, S35) were introgressed with six individual stay-green QTLs (from the donor parent B35; the development of ILs has been described in Kassahun et al. 2010 and Vadez et al. 2011).These ILs were used to explore the variation in several mechanisms involved in the plant water budget, and were expected to eventually lead to yield improvement under water stress and stay-green phenotype expression, which was further tested with the crop model (see below). Both of the senescent recipient lines as well as the stay-green QTL donor parent are considered to be limited-tillering materials. The stay-green donor was B35 (BT × 642) in both cases; B35 is a triple dwarf genotype.
Crop simulation approach – traits related to plant water budget and trait simulation
The sorghum (Sorghum bicolor (L.) Moench) model in APSIM (ver. 7.3; http://www.apsim.info/Products/Downloads.aspx; Keating et al. 2003; Hammer et al. 2010) and the original parameters developed for the common rabi-grown genotype M35–1 (Maldandi type; Ravi Kumar et al. 2009) were used to conduct simulations for the key locations within the five clusters of stress scenarios previously identified across the main rabi sorghum production tract (the simulations set is based on 404 years of historical weather records across 19 locations; the details are given in Kholová et al. 2013). These five stress scenarios were: (i) pre-flowering, (ii) flowering, (iii) post- flowering stress; (iv) stress relieved after flowering, and (v) no stress. Crop water demand is largely determined by the canopy size, the canopy conductance response to environmental stimuli (high evaporative demand of the air, soil water deficit) and the ability to extract water from the soil. Keeping how APSIM is structured in mind, our efforts focussed on the assessment of genetic variation in the set of stay-green QTL ILs in the R16 and S35 backgrounds for the production component traits related to (i) canopy development, (ii) capacity of the canopy and root to conduct water, and (iii) the canopy development response to water stress. The range of IL variations was then used to individually alter the crop parameters of the rabi-adapted sorghum variety M35–1 (as a ‘virtual introgression’ of individual traits), in a similar magnitude to that identified within the population of stay-green IL (i.e. original coefficient ± % variationmax). These were used to simulate their effects on stover and grain yield across the sorghum production tract with APSIM. Consequently, these virtual trait introgressions were compared with the original stover and grain yield simulations that were carried out with M35–1 parameters only (Kholová et al. 2013). The virtual crop growth was then analysed in more detail in seasons facing severe water limitations at a representative production site (Solapur) to visualise how the traits affected the stress patterns and the expression of the stay-green phenotype. Finally, several trait combinations and their effects on crop production, resilience and economic importance were investigated.
Leaf area growth dynamics and its simulation
Observed variation in canopy parameters
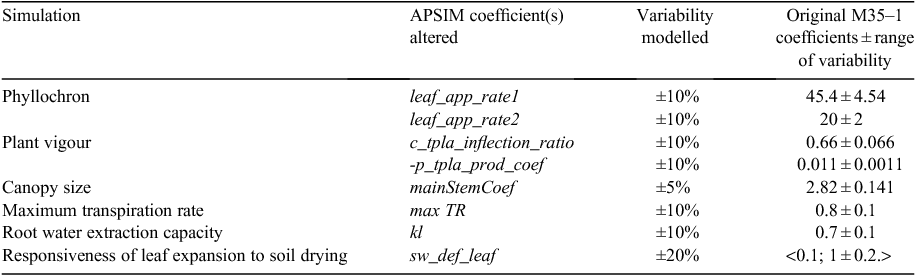
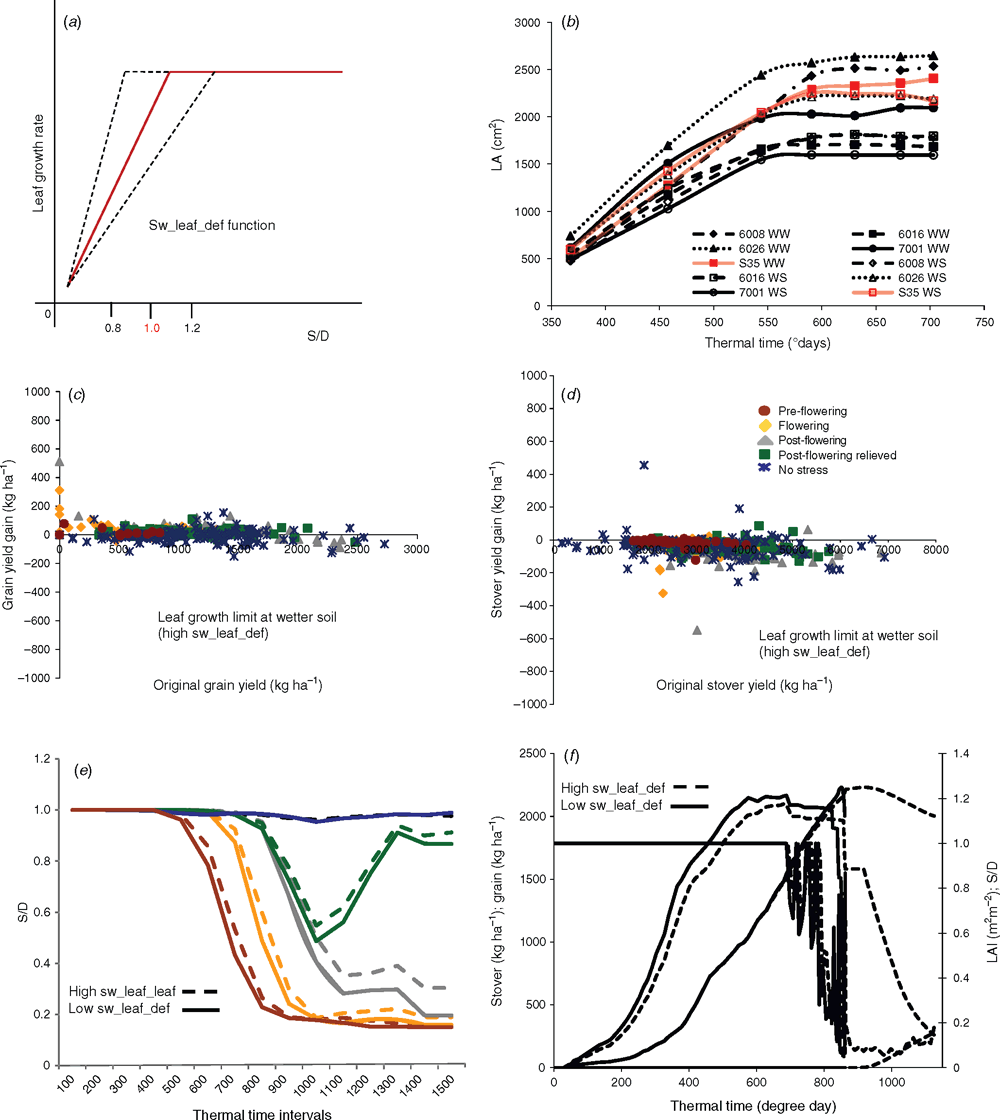
The growth of ILs was assessed under field conditions following standard methods for APSIM genotype parameterisation (Birch et al. 1990). This method builds on dynamic observations of plant phenological phases, leaf appearance during the season and leaf size distribution in plants grown under optimal conditions. The combination of these three parameters defined the canopy development as a function of thermal time and allow the estimation of APSIM coefficients to reflect the genotypic variability. Data were collected in field conditions during three seasons: 2010–11 (16 November to 20 March), 2011–12 (13 October to 10 February) and 2012–13 (8 November to 12 March). Field management and organisation were very similar to that of Kassahun et al. 2010. The analysis of 3 years of these field trials provided us with a range of variation in the corresponding parameters characterising crop canopy development in APSIM 7.3 within the population of ILs. The maximum observed variation in IL parameters (see below, Table 1 and Figs 1–3) was applied to change M35–1 coefficients in a similar range: γ ± 5% (2.82 ± 0.141); β ± 10% (0.66 ± 0.066); α ± 10% (0.011 ± 0.0011); Leaf Appearance Rate 1 ± 10% (45.5 ± 4.55); Leaf Appearance Rate 2 ± 10% (20 ± 2) (see below and Figs 1–3).
Simulation of canopy development
Canopy development is simulated on a whole-plant basis through a relationship between total plant leaf area (TPLA) and thermal time. TPLA integrates the number of fully expanded leaves, their individual size and tiller number, and includes an adjustment for the area of expanding leaves, as calculated by Eqns 1 and 2 (Hammer et al. 1993):


where TPLAmax is the maximum value of TPLA; TT was calculated from daily maximum and minimum temperatures as per Hammer and Muchow (1994); α, β and γ are fitted coefficients; FTN is fertile tiller number and TLN is total leaf (node) number. The value of β is usually set at 66% of the thermal time from emergence to flag leaf full expansion (Hammer et al. 1993). Variation in TPLAmax is associated with differences in TLN or γ (i.e. leaf size), resulting in the crop type attaining smaller or larger TPLA throughout the crop’s life cycle (Fig. 1a).
Variation of the other coefficients in the TPLA function
Variation of the other coefficients in the TPLA function (α and β, the TPLA production coefficient and the TPLA inflection ratio coefficient in APSIM, respectively) can be used to separate and mimic the effect of faster canopy development at earlier growth phases combined with slower canopy development in the later phases (plants with high ‘early vigour’) or vice versa, with initial slower and later increased canopy developmental rates (low ‘early vigour’). Both of these plant types (early or late vigour) would ultimately develop similar total leaf area at booting (given the same TPLAmax attributes) under optimal conditions (Fig. 2a), although with a different dynamic through the vegetative phase of the crop cycle. There are four hypothetical combinations shown on Fig. 2a but only two combinations were used for modelling – bold curves).
Simulation of leaf appearance rate
The number of fully expanded leaves is the product of thermal time elapsed since emergence and the leaf appearance rate. The rate of leaf appearance is characterised by a constant thermal time per leaf or phyllochron (Leaf Appearance Rate 1), except for the top 3.5 leaves, which appear at a more rapid rate (Leaf Appearance Rate 2). Although any change in phyllochron does not affect total leaf number, it does affect crop phenology, as the type with an extended (or reduced) phyllochron interval reaches full flag leaf expansion and thus flowering, later (or sooner) (Fig. 3a). Affecting the phyllochron parameters thus leads to changes in flowering time (short or long crop duration) while keeping the same canopy size attributes via TPLAmax and TPLA attributes.
Limited maximum transpiration rate under high vapour pressure deficit, soil water extraction rate and their simulation
Observed variation in maximum transpiration rate
The transpiration rate (TR, g H2O transpired cm–2 h–1) of well watered plants subjected to a range of vapour pressure deficit (VPD) regimes was measured in controlled environment growth chambers according to previous work (Kholová et al. 2010). The VPD regimes used in the assay represent the range of VPD conditions that plants usually face during rabi season cultivation. Ten ILs in both the R16 and S35 genetic backgrounds were assessed. There was limited variation in the S35 background but significant variation in the R16 background, which was further modelled (Fig. 3b).
Simulation of limits on maximum transpiration rate
The variation in this trait was simulated through APSIM by imposing a restriction on transpiration at the time of the day when potential crop water use is the highest. To do so, APSIM was adapted to calculate crop growth on an hourly basis, instead of a daily basis as in the standard model. This incorporated procedures to generate hourly weather variables from daily values (Hammer and Wright 1994; Glassy and Running 1994). A restriction on crop TR was then imposed in a manner similar to that of Sinclair et al. (2005) (Fig. 4a). The limit on the maximum TR was imposed at 0.8 (mm H2O m–2 of leaf area (LA) h–1] ± 0.1 (±10%), which reflected the variation in stay-green ILs (Table 1). Additionally, simulations of the limited maximum TR in combination with other traits investigated in this study were evaluated and are summarised in Table S1, available as Supplementary Material to this paper, but are not discussed in details.
Observed variability in soil water extraction rates
Recent research indicates that the restriction of transpiration under high VPD conditions could be of a hydraulic nature and linked to the extraction capacity of roots, for instance in barley (Hordeum vulgare L.), wheat (Triticum aestivum L.) or pearl millet (Pennisetum glaucum (L.) R. Br.) (Manschadi et al. 2006; Bramley et al. 2009; J. Kholová, M. Tharanya, S. Sakhti, unpubl. data). This possibility was then approached through an alteration of the kl coefficient in APSIM, which determines the soil water extraction rate (Robertson et al. 1993; Meinke et al. 1993). The differences in soil water extraction dynamics for various soil water levels were re-analysed from earlier data (Vadez et al. 2011) and different types of water use dynamic patterns were found among R16 ILs and S35 ILs. In R16 ILs, the total amount of water extracted from lysimeters differed little; however, there was variation in the dynamic of water extraction following irrigation withdrawal, suggesting variability in root hydraulics in ILs in the R16 background (Fig. 5b; the actual lysimeter weight is normalised by the field capacity lysimeter weight during the intervals after stress exposure). In S35 ILs, the total amount of water extracted from lysimeters differed among lines (Vadez et al. 2011), suggesting the involvement of different mechanisms in these S35 ILs and these were not investigated further here.
Simulation of soil water extraction rates
The APSIM kl constant determines the rate of soil water extraction as a function of soil moisture (Fig. 5a). It is an empirically derived coefficient representing a combination of soil–root hydraulic properties (k) and root length density (l) (Passioura 1983). Hence, it captures hydraulic limitation to water absorption by the crop. Apart from kl representing consequence of crop variability in root hydraulic conductance (Bramley et al. 2009; Sadok 2013; J. Kholová, M. Tharanya, S. Sakhti, unpubl. data), it can also reflect variations in root architecture (Manschadi et al. 2006). Kl and soil water content determine the potential supply of water to the crop for transpiration. This determines the crop water status via the transpiration supply : demand ratio, which drives the plant response factors that affect crop phenology, biomass production and LA development (Chapman et al. 1993; Hammer et al. 2010). Therefore, altering the kl coefficient simulates changes in the timing of water stress limitations on the crop. It was used here to mimic a possible change in root hydraulic conductance. The data on water extraction as a function of soil moisture from the ILs were fitted to changes in the kl coefficient (kl = 0.07 ± 0.01 (~10%); Fig. 5; Table 1).
Canopy sensitivity to declining soil water and its simulation
Observed variability in responsiveness of leaf expansion to stress
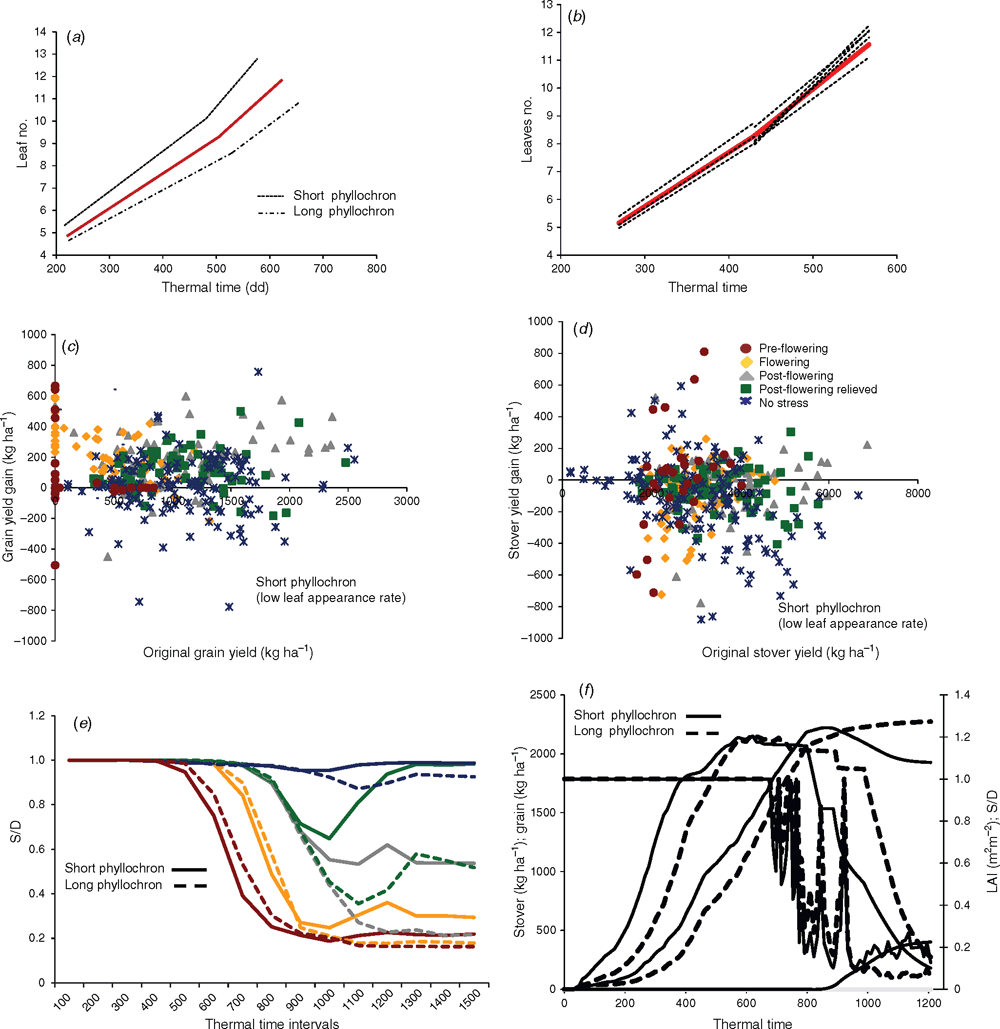
A decline in leaf expansion at high soil moisture levels would save water and would potentially be an important plant feature for drought adaptation, although it would also restrict the plant leaf area and eventually light interception and biomass accumulation (Chenu et al. 2008, 2009). Here, the different ILs were grown in lysimeters during two seasons (2010–11 and 2011–12) and exposed to terminal drought as described earlier (Vadez et al. 2011). Several times during the advancing water stress, the canopy leaf area was nondestructively assessed (LA was equal to leaf length × width × 0.69). The genotypic variability in the response of leaf area development to soil drying was expressed by measuring the LA difference from a fully irrigated control after cessation of watering (Fig. 6b).
Simulation of responsiveness of leaf expansion to stress
The differences in the sensitivity of leaf expansion to drought were approached through alterations in the stress response coefficients (sw_def_leaf) of APSIM. The sw_def_leaf coefficients quantify the range of crop water status via the transpiration supply : demand ratio over which reduction in leaf growth is imposed. Therefore, a higher upper limit of this supply–demand window (<0.1; 1.2>), instead of the standard window (<0.1, 1.0>), would slow down leaf growth sooner (e.g. at higher soil moisture). This range in the sw_def_leaf coefficients was assessed from the ILs measured behaviour and its variation estimated (<0.1; 1 ± 0.2> (±20%); Fig. 6).
Statistical analysis
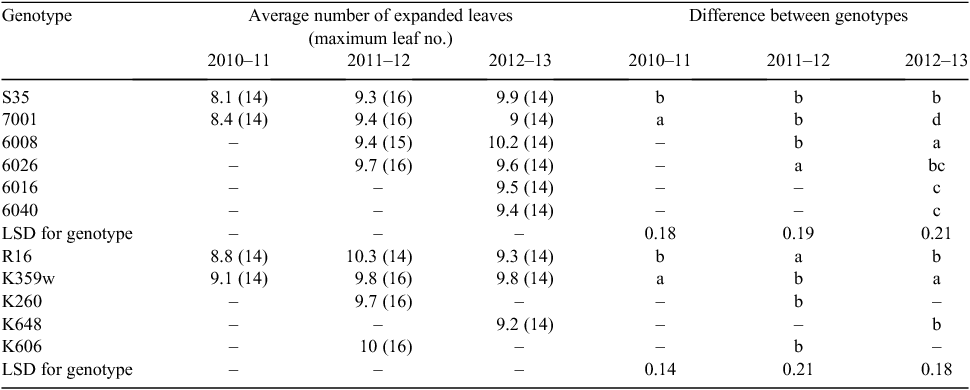
Transpiration rate response to VPD was analysed via a broken linear model using Genstat (VSN International, Hemel Hempstead, UK) and visualised using GraphPrismPad ver. 6.1 (GraphPad Software, San Diego, CA, USA). Canopy development parameters (i.e. leaf size and leaf appearance) were analysed using a two-way randomised block ANOVA (with leaf numbers and weekly leaf counts as blocks; Table 2) across seasons and genotypes to illustrate the influence of season, genotype and genotype × season interactions. We further used a one-way block ANOVA to analyse the genotypic differences of leaf appearance within the season and the effect of season within genotype (Tables 3, 4).

|
Simulation outputs were analysed within the stress scenarios defined by Kholová et al. (2013). This work is intended to assist sorghum breeding programs in which the final economic value of the trait is of central importance. Therefore, a preliminary attempt was made to estimate the economic value of each mechanism for each stress pattern, in order to explore economic trade-offs, knowing that the usual stover price is around one-third (worst quality) to half (best quality) of the grain price. In this study, we used an average fixed price of ₹15 per kg for grain and ₹5 per kg for stover (Directorate of Marketing & Inspection 2014). These results are summarised in Table S1 but are not discussed in detail.
Results
Leaf area growth dynamics and its simulation
Observed variation in canopy parameters
The two-way ANOVA of three seasons of field data showed a significant effect of season and genotype on leaf size and a significant genotype × season interaction effect (Tables 2, 3). Significant differences in the rates of leaf appearance between genotypes were also found (Table 4, Fig. 3b) and were accompanied by variation in flowering time (± ~5 days, data not shown). This variability was reflected in the derivation of APSIM coefficients.
Simulation of TPLAmax
A simulated smaller canopy size is predicted to improve yield in all water stress environments of the rabi tract except for the limited stress or unstressed scenarios (Table S1, Fig. 1c). At the same time, less canopy development decreased the expected stover yield across all stress scenarios (Table S1, Fig. 1d). Simulation of larger canopy size had the opposite effect on predictions of grain and stover production (Table S1). A smaller canopy size delayed the onset of stress in terminal stress scenarios (Fig. 1e). It also led to a grain yield advantage, which was caused by a delay in water exhaustion during the season. This shift in water usage also delayed leaf senescence and led to an expression of a virtual stay-green phenotype (Fig. 1f).
Simulation of plant vigour (TPLA coefficients α and β)
Simulation of plants with high early vigour showed a grain yield penalty in all environments except in the unstressed scenario, but it led to higher stover production across all scenarios (Table S1). On the contrary, plants with low early vigour led to a mild grain yield production increase in all stress scenarios, except the unstress scenario, accompanied by a mild loss in stover production in all environments (Table S1; Fig. 2c, d). This also led to water conservation, as in the case discussed above (Fig. 2 e, f). Overall, altering the TPLA inflection coefficient and the production ratio had only mild effects on the evolution of the stress patterns (Fig. 2e, f), and very small effect on grain and stover yield (Fig. 2c, d), although it appeared to increase yield stability (Table S1).
Simulation of phyllochron (Leaf Appearance Rate 1 and 2)
Simulations of longer crop duration via longer phyllochron intervals decreased grain yield in all stress scenarios, although it increased stover yield to a similar magnitude except for in the preflowering stress scenario (Table S1). Shortening the phyllochron interval and therefore crop duration substantially increased the predicted grain yield, although it decreased stover production (Fig. 4c, d; Table S1). Here, the estimated decrease in stover yield (in kg ha–1) was of a smaller magnitude than the gain in grain yield. In the most severe stress scenarios, the shorter duration crop exhausted water resources faster. However, it improved water use after anthesis in less severe stress scenarios (Fig. 3e). Under severe stress, the crops with shorter phyllochron intervals had a larger part of their preflowering phase taking place under no stress conditions, compared with the crop with a longer phyllochron (Fig. 3f). The crops with a short phyllochron has grown the LA earlier and also exhausted the available water and senesced before the crops with a longer phyllochron. Thus, in this specific case, the differences relate to avoiding water limitation at critical stages rather than water conservation.
Limited maximum TR and soil water extraction rate, and their simulation
Observed variation in limits on maximum TR
The rates of transpiration upon exposure to a range of VPD conditions varied between the senescent parental lines and the ILs especially within the ILs in the R16 background, with either higher or lower TR than R16 (Fig. 4b). There was little variation among ILs in the S35 background (data not shown) and therefore the variation within R16 ILs was used to guide the modelling of these effects.
Simulation of limits on maximum TR
Setting the limits on the TR by ~10% in M35–1-generated crops with a high probability of increasing both the grain and stover yields across all stress scenarios (Fig. 4c, d). This implies that, in contrast to all the other traits tested in this paper, the limited transpiration trait did not involve production trade-offs between grain and stover but increased overall water productivity (TE). The lowest yield gains were predicted within the unstressed scenario. Limiting the maximum TR in the severe stress scenarios restored yield due to improved water use after anthesis, in several cases where there was otherwise a crop failure. However, the delay in the onset of stress due to the limited TR was small compared with other traits (Fig. 4e) but was sufficient for expression of the stay-green phenotype (Fig. 4f).
Observed variability in the soil water extraction rate
Reanalysis of data from Vadez et al. (2011) showed substantial genetic variability in the rates of water extraction especially in R16-derived ILs (Fig. 5b). This variability was estimated to be in the range of ± ~10% from the reference senescent parental line (R16) and justified further simulation analysis.
Simulation of soil water extraction rate
The virtual M35–1 crop with lower water extracting capacity at declining soil moisture (lower kl) realised a modest increase in stover yield in most of the stress scenarios but maintained similar grain yield levels (Table S1; Fig. 5c, d). However, there was large variability in the kl effect under particular stress scenarios (Fig. 5c, d). The plants with a lower kl entered the water stress slightly earlier but were able to extend the duration of water availability (due to a simultaneous slow-down in biomass accumulation). This was especially notable in the scenarios of stress imposed after flowering and relieved stress after flowering (Figs 4, 5). Also, in the severe stress situation, the low-kl crop had slightly improved TE by limiting supply to the crop. So, although limitations in root extraction generated slightly early stress onset, they resulted in an extended period for which growth was maintained, which would contribute to the improved crop resilience and potential for stay-green expression (Fig. 5f).
Canopy sensitivity to declining soil water and its simulation
Observed variability in responsiveness of leaf expansion to stress
Upon stopping irrigation at 5 weeks after sowing, most of the ILs restricted their leaf expansion upon exposure to soil drying, whereas their respective senescent parents developed similar LA as they did in the fully-irrigated treatment (Fig. 6b shows variability in S35 ILs, although there was a similar range of variability in R16 ILs).
Simulation of responsiveness of leaf expansion to stress
The simulated slow-down of leaf expansion in drier soil (broadening the sw-leaf-def interval) had little effect on yield across all environments, whereas it increased stover productivity across all environments (Table S1; Fig. 6c, d). Generally, a crop with a decline in leaf expansion in wetter soil slightly postponed the onset of stress and improved water extraction during the grain filling stage, especially in the case of the intermittent stress scenario (Fig. 6e). Furthermore, the crops restricting leaf expansion in wetter soil, under severe water stress, had a higher grain yield than crops with a decline in leaf expansion decline in drier soil (Fig. 6c–f). The failure of the crop with decreased leaf expansion in drier soil could be explained by its enhanced biomass accumulation during the early stages of growth and larger leaf area development followed by a restricted period of water use after anthesis which can therefore also lead to earlier senescence (Fig. 6f). In any case, different sensitivities of leaf expansion to stress had only small effects on grain and stover yield.
Economic value of traits
The proportion of various stress scenarios differs within the rabi sorghum production zones (defined in Kholová et al. 2013); therefore, the importance of the traits for use in breeding programs should be weighted accordingly. Initial insights into the putative breeding value of the investigated traits (and some of their combinations) within production zones are shown in Table S1 but the data are not discussed.
Discussion
In this work, we have built up on previous modelling that characterises of stress environments across major rabi sorghum production zones (details in Kholová et al. 2013) to assess the effect of traits related to the plant water budget on grain and stover yield. Several traits postponed the onset of stress and increased water use after anthesis, which is phenotypically expressed as delayed leaf senescence and led to a virtual stay-green phenotype, although other traits also affected TE. Maintenance of green leaf area generally led to improved grain productivity, although, in most scenarios, an increase in grain productivity was counterbalanced by a decrease in stover productivity and vice versa. The improvement of both grain and stover productivity was possible only through improvement of plant water productivity (TE) and was a result of simulating a restricted crop TR under high VPD. From a breeding point of view, a decreased TR under high VPD would be the most worthwhile strategy for crop drought adaptation improvement. Apart from a reduced TR, any other way of improving stover productivity would increase the risk of grain yield failure and vice versa; an increase in grain productivity was feasible only in crops with supressed stover production. In short, we identified a set of physiological processes leading to the expression of the stay-green phenotype across the rabi sorghum belt that could improve grain and stover yield and their stability under current management practices. This knowledge allows us to construct crop ideotypes for particular locations, according to the proportion of stress scenarios and according to farmers’ specific demands (for either stover or grain).
Observed variability in traits affecting plant water use
Introgression of stay-green QTLs led to either lower or higher canopy size in ILs. However, significant genotype × season interactions, especially between earlier (October 2011–12) and later plantings (November 2010–11 and 2012–13), indicated that variability in photoperiod or temperature sensitivity could have affected canopy development (e.g. Bos and Neuteboom 1998; Kim et al. 2010a, 2010b; van Oosterom et al. 2011). Leaf expansion is also known to be sensitive to VPD (e.g. Reymond et al. 2003; Welcker et al. 2007) and VPD differences across season could have also affected the canopy development. Therefore, more work is needed to more thoroughly assess how VPD could have altered the leaf development coefficient, and also to develop the necessary loops in APSIM to reflect genetic differences in these effects. In addition, various ILs from both backgrounds (R16 and S35) limited leaf expansion upon soil drying, pointing to a genetic mean of modulating leaf area if drought prevails during the vegetative stages. Additional work is therefore needed to properly characterise how VPD and soil moisture affect the leaf canopy development across the different stay-green QTL introgressions.
There is much research describing the genotypic variability in canopy conductance under high VPD, related, in some cases, to the hydraulic properties of plant tissues (e.g. Fletcher et al. 2008; Bramley et al. 2009; Devi et al. 2010; Kholová et al. 2010a, 2010b; Gholipoor et al. 2010) and resulting in differences in crop water use (e.g. Vadez et al. 2014). There are also modelling exercises predicting benefits of limited crop hydraulics in water-limited environments (Sinclair and Hammer et al. 2005; Manschadi et al. 2006; Sinclair et al. 2010). Here, we have documented that QTL introgressions indeed influence plant canopy water conductivity and reduced or enhanced TR, especially under high VPD, although this was specific to the R16 background. This observation confirms that a given physiological mechanism may not be simply transferable to all genetic backgrounds and may depend on the genetic context of the recurrent parent (similarly in Vadez et al. 2011). In addition to these, differences in soil water absorption capacity were found among the ILs. Whether these were related to the differences in TR is unknown. In other studies, differences in TR were, in fact, linked to the difference in soil water absorption capacity (e.g. Bramley et al. 2009; Sadok 2013). In any case, our observations showed that stay-green QTLs could alter the rate of water loss by the canopy, or the rate of water absorption by the roots.
Traits affecting plant water use can lead to water saving and the stay-green phenotype
Alterating of the canopy development parameters in APSIM altered stress patterns and led to the expression of a stay-green phenotype. Grain yield improvement in the water stress scenarios came from constitutive water conservation during vegetative growth via reduced canopy size during the entire growth period or particular phases of growth. An exception was the alteration of the phyllochron, resulting in different crop durations. In this case, in the severe stress scenario, the short-duration crop senesced and exhausted the water earlier but also escaped drought with a larger portion of its preanthesis period taking place under unstressed conditions. Thus, in this particular case the stay-green expression was linked to a long-duration genotype with less grain yield produced at the end of the season. This shows clearly that the stay-green phenotype under drought and plant canopy development are closely linked and the current APSIM model is adequate to mimic these relationships. Surprisingly, these relations between canopy development and stay-green were not seriously explored until quite recently (Hammer 2006; van Oosterom et al. 2011; Borrell 2013; Vadez et al. 2014). In fact, only a few reports on sorghum experimentally documented similar effects of canopy development (phyllochron, tillering) on crop production (van Oosterom et al. 2010a, 2010b; Borrell 2013).
Lower canopy TR under high VPD conditions and low rates of soil water extraction also led to stay-green expression in the severe water limited environments. In these two cases, stay-green expression in the crops also appeared to be linked to saving the water in the soil profile but to a lesser extent compared with crops with altered canopy growth (see above). In the case of low TR, the amount of water saved depended on the VPD throughout the day and the benefit mostly came from an increased TE rather than from a shift in water use before and after anthesis.
Canopy growth traits resulted in grain–stover production trade-offs
Alteration in the plant canopy traits saved water and increased yield, especially in the severe scenarios, but it resulted in trade-offs between grain and stover productivity, depending on the stress scenario. Also, increased soil water extraction rates (high kl) of the crop improved stover accumulation but resulted in a trade-off with grain production, similar to the case of manipulating the canopy parameters. To use these traits in breeding, the crop grain and stover production benefits from canopy-related mechanisms would need to be weighted by the frequencies of a particular stress scenario in the target location so as to maximise the economic gains (as shown in Table S1). Our results indicate that particularly large economic gains (large gains in grain yield compared with minute stover yield losses) can come from shortening the crop duration (short phyllochron intervals) or from developing a smaller canopy size. The easiest and most effective breeding target should be the optimisation of crop duration within the production zones, upon which other traits can be further built. We also showed that generally, crops with higher stover productivity increase their chances of grain yield failure in severe stress environments. Therefore, our findings can be used not only to weight the production benefits but also leave the choice to the farmers themselves. If the current demand requires farmers to produce more biomass (e.g. as cattle fodder) with marginal interest in grain or vice versa, we could tailor or recommend the crop type that fits these particular needs. Additionally, the differences in canopy growth and development may also impact the grain and stover yield quality, which would affect the grain and stover prices (Blümmel and Rao 2006) but this variability has not yet been characterised sufficiently.
Restriction of TR under high VPD and lower soil water extraction rate improve TE, and grain and stover production
In contrast to canopy growth traits, which always led to trade-offs between grain and stover productivity, lower TR improved stover and grain production at the same time across most of the stress scenarios and so is expected to have high economic potential. This was possible only because the low TR crop had enhanced TE. The benefit for grain yield did not come only from a mere shifting in plant water use from before to after anthesis.
Decreased soil water extraction capacity (through kl) generated crops with a high variability in production benefits or losses within the particular stress scenario and had no major effect on the average crop production. Nevertheless, crops with lower soil water extraction capacity have the potential to enhance TE, which seems to improve the crops’ resilience under severe stress scenarios. This is because reducing the roots’ kl coefficient mimics a plant that enters more quickly into water stress but can maintain growth for longer (with increased TE at low soil moisture levels). Here again, the alteration of the kl coefficient was chosen as a means of altering root hydraulic conductance. However, the APSIM structure is such that altering the kl coefficient has other ‘pleiotropic’ effects on other plant development aspects and we would suggest that further work is needed in APSIM to improve the water capture loops in order to sensibly reproduce the crop variability presented here.
Conclusions
In this work, we demonstrated in silico that the variability in the traits and processes related to plant water use (canopy development, capacity of the canopy and root to conduct water and the canopy development response to water stress) improved grain yield and led to stay-green expression as a consequence of increased water availability after anthesis. Improvements in grain yield showed negative stover production trade-offs but improved crop production stability across years. Simultaneous improvement of grain and stover yield along with crop resilience was possible only through enhancing water productivity (TE) and was the result of imposing limits on canopy transpiration under high VPD (TR). Therefore, this study provides a range of tools for constructing of zone- and demand-specific crop ideotypes that may be considered in breeding schemes. More work is underway to assess how alteration in management practices could affect stay-green expression, water use patterns and crop production. The present work provides a solid base to investigate these genotype × management interactions further.
Acknowledgements
The authors acknowledge support from the CGIAR Research Program on Dryland Cereals, the Research Program on Climate Change, Agriculture and Food Security, and the Australian Center for International Agriculture Research (CIM-2007–120), which have supported some of the research activities presented in this article.
References
Bertheloot J, Martre P, Andrieu B (2008) Dynamics of light and nitrogen distribution during grain filling within wheat canopy. Plant Physiology 148, 1707–1720.| Dynamics of light and nitrogen distribution during grain filling within wheat canopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVSnurbE&md5=0524d2836726b29a03c70b479e955c0cCAS | 18799664PubMed |
Bidinger FR, Nepolean T, Hash CT, Yadav RS, Howarth CJ (2007) Identification of QTLs for grain yield of pearl millet [Pennisetum glaucum (L.) R. Br.] in environments with variable moisture during grain filling. Crop Science 47, 969–980.
| Identification of QTLs for grain yield of pearl millet [Pennisetum glaucum (L.) R. Br.] in environments with variable moisture during grain filling.Crossref | GoogleScholarGoogle Scholar |
Birch CJ, Carberry PS, Muchow RC, McCown RL, Hargreaves JNG (1990) Development and evaluation of a sorghum model based on CERES-Maize in a semi-arid tropical environment. Field Crops Research 24, 87–104.
| Development and evaluation of a sorghum model based on CERES-Maize in a semi-arid tropical environment.Crossref | GoogleScholarGoogle Scholar |
Blümmel M, Rao PP (2006) Economic value of sorghum stover traded as fodder for urban and peri-urban dairy production in Hyderabad, India. (Patancheru: International Crops Research Institute for the Semi-Arid Tropics) Available online at: http://ejournal.icrisat.org/mpii/v2i1/v2i1economicvalue.pdf [Verified 17 June 2014]
Borrell AK (2013) Fine-mapping candidates for ‘stay-green’ in sorghum reveals genes associated with canopy development and root growth. In ‘Proceedings of Interdrought IV, Perth’. (Eds R. Tuberosa, N. Turner, M. Cakir) p. 147. (EECW: Perth)
Borrell AK, Hammer GL (2000) Nitrogen dynamics and the physiological basis of stay-green in sorghum. Crop Science 40, 1295–1307.
| Nitrogen dynamics and the physiological basis of stay-green in sorghum.Crossref | GoogleScholarGoogle Scholar |
Borrell A, Hammer G, van Oosterom E (2001) Stay-green: a consequence of the balance between supply and demand for nitrogen during grain filling. Annals of Applied Biology 138, 91–95.
| Stay-green: a consequence of the balance between supply and demand for nitrogen during grain filling.Crossref | GoogleScholarGoogle Scholar |
Bos HJ, Neuteboom JH (1998) Morphological analysis of leaf and tiller number dynamics of wheat (Triticum aestivum L.): responses to temperature and light intensity. Annals of Botany 81, 131–139.
| Morphological analysis of leaf and tiller number dynamics of wheat (Triticum aestivum L.): responses to temperature and light intensity.Crossref | GoogleScholarGoogle Scholar |
Bramley H, Turner NC, Turner DW, Tyerman SD (2009) Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behaviour of roots. Plant Physiology 150, 348–364.
| Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behaviour of roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlvFahsL8%3D&md5=3738b572044af73ca546fc0257626b23CAS | 19321713PubMed |
Cha KW, Lee YJ, Koh HJ, Lee BM, Nam YW, Paek NC (2002) Isolation, characterization, and mapping of the stay green mutant in rice. Theoretical and Applied Genetics 104, 526–532.
| Isolation, characterization, and mapping of the stay green mutant in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjtlSqt74%3D&md5=80e627da54aec728b35cb014c39244c6CAS | 12582654PubMed |
Chapman SC, Hammer GL, Meinke H (1993) A sunflower simulation model: I. Model development. Agronomy Journal 85, 725–735.
| A sunflower simulation model: I. Model development.Crossref | GoogleScholarGoogle Scholar |
Chapman SC, Cooper M, Butler DG, Henzel RG (2000a) Genotype by environment interactions affecting grain sorghum. I. Characteristics that confound interpretation of hybrid yield. Crop and Pasture Science 51, 197–208.
| Genotype by environment interactions affecting grain sorghum. I. Characteristics that confound interpretation of hybrid yield.Crossref | GoogleScholarGoogle Scholar |
Chapman SC, Cooper M, Hammer GL, Butler DG (2000b) Genotype by environment interactions affecting grain sorghum. II. Frequencies of different seasonal patterns of drought stress are related to location effects on hybrid yields. Australian Journal of Agricultural Research 51, 209–221.
| Genotype by environment interactions affecting grain sorghum. II. Frequencies of different seasonal patterns of drought stress are related to location effects on hybrid yields.Crossref | GoogleScholarGoogle Scholar |
Chapman SC, Hammer GL, Butler DG, Cooper M (2000c) Genotype by environment interactions affecting grain sorghum. III. Temporal sequences and spatial patterns in the target population of environments. Australian Journal of Agricultural Research 51, 223–234.
| Genotype by environment interactions affecting grain sorghum. III. Temporal sequences and spatial patterns in the target population of environments.Crossref | GoogleScholarGoogle Scholar |
Chauhan Y, Wright G, Rachaputi N, McCosker K (2008) Indentifying chickpea homoclimes using the APSIM chickpea model. Australian Journal of Agricultural Research 59, 260–269.
| Indentifying chickpea homoclimes using the APSIM chickpea model.Crossref | GoogleScholarGoogle Scholar |
Chauhan Y, Solomon KF, Rodriguez D (2013) Characterization of north-eastern Australian environments using APSIM for increasing rain-fed maize production. Field Crops Research 144, 245–255.
| Characterization of north-eastern Australian environments using APSIM for increasing rain-fed maize production.Crossref | GoogleScholarGoogle Scholar |
Chenu K, Chapman SC, Hammer GL, McLean G, Tardieu F (2008) Short term responses of leaf growth rate to water deficit scale up to whole plant and crop levels. An integrated modelling approach in maize. Plant, Cell & Environment 31, 378–391.
| Short term responses of leaf growth rate to water deficit scale up to whole plant and crop levels. An integrated modelling approach in maize.Crossref | GoogleScholarGoogle Scholar |
Chenu K, Chapman SC, Tardieu F, McLean G, Welcker C, Hammer GL (2009) Simulating the yield impacts of organ-level quantitative trait loci associated with drought response in maize – a ‘gene-to-phenotype’ modeling approach. Genetics 183, 1507–1523.
| Simulating the yield impacts of organ-level quantitative trait loci associated with drought response in maize – a ‘gene-to-phenotype’ modeling approach.Crossref | GoogleScholarGoogle Scholar | 19786622PubMed |
Chenu K, Cooper M, Hammer GL, Mathews KL, Dreccer MF, Chapman SC (2011) Environment characterization as an aid to wheat improvement: interpreting genotype–environment interactions by modelling water-deficit patterns in north-eastern Australia. Journal of Experimental Botany 62, 1743–1755.
| Environment characterization as an aid to wheat improvement: interpreting genotype–environment interactions by modelling water-deficit patterns in north-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFyis7w%3D&md5=ce5813d9210984f6cb22146b1fbde110CAS | 21421705PubMed |
Chenu K, Deihimfard R, Chapman SC (2013) Large-scale characterization of drought pattern: a continent-wide modelling approach applied to the Australian wheatbelt – spatial and temporal trends. New Phytologist 198, 801–820.
| Large-scale characterization of drought pattern: a continent-wide modelling approach applied to the Australian wheatbelt – spatial and temporal trends.Crossref | GoogleScholarGoogle Scholar | 23425331PubMed |
Crasta OR, Xu WW, Rosenow DT, Mullet JE, Nguyen HT (1999) Mapping of post-flowering drought resistance traits in grain sorghum: association of QTLs in premature senescence and maturity. Molecular & General Genetics 262, 579–588.
| Mapping of post-flowering drought resistance traits in grain sorghum: association of QTLs in premature senescence and maturity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnvFKrt78%3D&md5=65d8a356cdcbcd8e429221a42f0bcfc5CAS | 25055336PubMed |
Devi JM, Sinclair TR, Vadez V (2010) Genotypic variation in peanut (Arachis hypogea L.) for transpiration sensitivity to atmospheric vapor pressure deficit. Crop Science 50, 191–196.
| Genotypic variation in peanut (Arachis hypogea L.) for transpiration sensitivity to atmospheric vapor pressure deficit.Crossref | GoogleScholarGoogle Scholar |
Directorate of Marketing and Inspection (DMI) (2014) Agmarknet. New Delhi: DMI, Ministry of Agriculture, Government of India; 2014. Available online at: http://agmarknet.nic.in/ (verified 17 June 2014).
Fletcher AL, Sinclair TR, Allen LH (2008) Vapor pressure deficit effects on leaf area expansion and transportation of soybean subjected to soil drying. Proceedings – Soil and Crop Science Society of Florida 67, 15–20.
Gholipoor M, Vara Prasad PV, Mutava RN, Sinclair TR (2010) Genetic variability of transpiration response to vapour pressure deficit among sorghum genotypes. Field Crops Research 119, 85–90.
| Genetic variability of transpiration response to vapour pressure deficit among sorghum genotypes.Crossref | GoogleScholarGoogle Scholar |
Glassy JM, Running SW (1994) Validating diurnal climatology logic of the MT-CLIM model across a climatic gradient in Oregon. Ecological Applications 4, 248–257.
| Validating diurnal climatology logic of the MT-CLIM model across a climatic gradient in Oregon.Crossref | GoogleScholarGoogle Scholar |
Hammer G (2006) Pathways to prosperity: breaking the yield barrier in sorghum. Agricultural Science 19, 16–22.
Hammer GL, Muchow RC (1994) Assessing climatic risk to sorghum production in water-limited subtropical environments. I. Development and testing of a simulation model. Field Crops Research 36, 221–234.
| Assessing climatic risk to sorghum production in water-limited subtropical environments. I. Development and testing of a simulation model.Crossref | GoogleScholarGoogle Scholar |
Hammer GL, Wright GC (1994) A theoretical analysis of nitrogen and radiation effects on radiation use efficiency in peanut. Australian Journal of Agricultural Research 45, 575–589.
| A theoretical analysis of nitrogen and radiation effects on radiation use efficiency in peanut.Crossref | GoogleScholarGoogle Scholar |
Hammer GL, Carberry PS, Muchow RC (1993) Modelling genotypic and environmental control of leaf area dynamics in grain sorghum. I. Whole plant level. Field Crops Research 33, 293–310.
| Modelling genotypic and environmental control of leaf area dynamics in grain sorghum. I. Whole plant level.Crossref | GoogleScholarGoogle Scholar |
Hammer GL, van Oosterom E, McLean G, Chapman SC, Broad I, Harland P, Muchow RC (2010) Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops. Journal of Experimental Botany 61, 2185–2202.
| Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVGnsr0%3D&md5=b2fef2f80ec556ab93571b718748923aCAS | 20400531PubMed |
Harris K, Subudhi PK, Borrell A, Jordan D, Rosenow D, Nguyen H, Klein P, Klein R, Mullet J (2007) Sorghum stay-green QTL individually reduce post-flowering drought-induced leaf senescence. Journal of Experimental Botany 58, 327–338.
| Sorghum stay-green QTL individually reduce post-flowering drought-induced leaf senescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlOlt7c%3D&md5=62753958637fa0a1475d31dab7b0f0feCAS | 17175550PubMed |
Hash CT, Bhasker Raj AG, Lindup S, Sharma A, Beniwal CR, Folkertsma RT, Mahalakshmi V, Zerbini E, Blümmel M (2003) Opportunities for marker-assisted selection (MAS) to improve the feed quality of crop residues in pearl millet and sorghum. Field Crops Research 84, 79–88.
| Opportunities for marker-assisted selection (MAS) to improve the feed quality of crop residues in pearl millet and sorghum.Crossref | GoogleScholarGoogle Scholar |
Haussmann BIG, Mahalakshmi V, Reddy BVS, Seetharama N, Hash CT, Geiger HH (2002) QTL mapping of stay-green in two sorghum recombinant inbred populations. Theoretical and Applied Genetics 106, 133–142.
Jordan DR, Tao T, Godwin ID, Henzell RG, Cooper M, McIntyre CL (2003) Prediction of hybrid performance in grain sorghum using RFLP markers. Theoretical and Applied Genetics 106, 559–567.
Kassahun B, Bidinger FR, Hash CT, Kuruvinashetti MS (2010) Stay-green expression in early generation sorghum (Sorghum bicolor (L.) Moench) QTL introgression lines. Euphytica 172, 351–362.
| Stay-green expression in early generation sorghum (Sorghum bicolor (L.) Moench) QTL introgression lines.Crossref | GoogleScholarGoogle Scholar |
Keating BA, Carberry PS, Hammer GL, Probert ME, Robertson MJ, Holzworth D, Huth NI, Hargreaves JNG, Meinke H, Hochman Z, McLean G, Verburg K, Snow V, Dimes JP, Silburn M, Wang E, Brown S, Bristow KL, Asseng S, Chapman S, McCown RL, Freebairn DM, Smith CJ (2003) An overview of APSIM, a model designed for farming systems simulation. European Journal of Agronomy 18, 267–288.
| An overview of APSIM, a model designed for farming systems simulation.Crossref | GoogleScholarGoogle Scholar |
Kebede H, Subudhi PK, Rosenow DT, Nguyen HT (2001) Quantitative trait loci infuencing drought tolerance in grain sorghum (Sorghum bicolor (L.) Moench). Theoretical and Applied Genetics 103, 266–276.
| Quantitative trait loci infuencing drought tolerance in grain sorghum (Sorghum bicolor (L.) Moench).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvVeks7c%3D&md5=31818d2578e9f6527aee9b4c49f51232CAS |
Kholová J, Hash CT, Kočová M, Vadez V (2010a) Constitutive water conserving mechanisms are correlated with the terminal drought tolerance of pearl millet (Pennisetum glaucum (L.) R. Br.). Journal of Experimental Botany 61, 369–377.
| Constitutive water conserving mechanisms are correlated with the terminal drought tolerance of pearl millet (Pennisetum glaucum (L.) R. Br.).Crossref | GoogleScholarGoogle Scholar | 19861657PubMed |
Kholová J, Hash CT, Kumar LK, Yadav RS, Kočová M, Vadez V (2010b) Terminal drought tolerant pearl millet (Pennisetum glaucum (L.) R. Br.) have high leaf ABA and limit transpiration at high vapor pressure deficit. Journal of Experimental Botany 61, 1431–1440.
| Terminal drought tolerant pearl millet (Pennisetum glaucum (L.) R. Br.) have high leaf ABA and limit transpiration at high vapor pressure deficit.Crossref | GoogleScholarGoogle Scholar | 20142425PubMed |
Kholová J, McLean G, Vadez V, Craufurd P, Hammer GL (2013) Drought stress characterization of post-rainy season (rabi) sorghum in India. Field Crops Research 141, 38–46.
| Drought stress characterization of post-rainy season (rabi) sorghum in India.Crossref | GoogleScholarGoogle Scholar |
Kim HK, Luquet D, van Oosterom E, Dingkuhn M, Hammer G (2010a) Regulation of tillering in sorghum: genotypic effects. Annals of Botany 106, 69–78.
| Regulation of tillering in sorghum: genotypic effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVWjsbs%3D&md5=74ed1e6dc392740a28778bef4bc35119CAS | 20430784PubMed |
Kim HK, van Oosterom E, Dingkuhn M, Luquet D, Hammer GL (2010b) Regulation of tillering in sorghum: environmental effects. Annals of Botany 106, 57–67.
| Regulation of tillering in sorghum: environmental effects.Crossref | GoogleScholarGoogle Scholar | 20421230PubMed |
Manschadi AM, Christopher J, deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33, 823–837.
| The role of root architectural traits in adaptation of wheat to water-limited environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XptVClsbY%3D&md5=a04db73a587e35eaf30b1da63aa5f120CAS |
Meinke H, Hammer GL, Want P (1993) Potential soil water extraction by sunflower on a range of soils. Field Crops Research 32, 59–81.
| Potential soil water extraction by sunflower on a range of soils.Crossref | GoogleScholarGoogle Scholar |
Murty MVR, Singh P, Wani SP, Khairwal IS, Srinivas K (2007) ‘Yield gap analysis of sorghum and pearl millet in India using simulation modeling. Global theme on agroecosystems report no. 37.’ (International Crops Research Institute for the Semi-Arid Tropics: Patancheru)
Passioura JB (1983) Roots and drought resistance. Agricultural Water Management 7, 265–280.
| Roots and drought resistance.Crossref | GoogleScholarGoogle Scholar |
Rajcan I, Tollenaar M (1999) Source: sink ratio and leaf senescence in maize: II. Nitrogen metabolism during grain filling. Field Crops Research 60, 255–265.
| Source: sink ratio and leaf senescence in maize: II. Nitrogen metabolism during grain filling.Crossref | GoogleScholarGoogle Scholar |
Ravi Kumar S, Hammer GL, Broad I, Harland P, McLean G (2009) Modelling environmental effects on phenology and canopy development of diverse sorghum genotypes. Field Crops Research 111, 157–165.
| Modelling environmental effects on phenology and canopy development of diverse sorghum genotypes.Crossref | GoogleScholarGoogle Scholar |
Reymond M, Muller B, Leonardi A, Charcosset A, Tardieu F (2003) Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of leaf growth to temperature and water deficit. Plant Physiology 131, 664–675.
| Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of leaf growth to temperature and water deficit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtlyjs7w%3D&md5=bbf0f3ff4d88be93e35863f8b856d7fcCAS | 12586890PubMed |
Robertson MJ, Fukai S, Ludlow MM, Hammer GL (1993) Water extraction by grain sorghum in a sub-humid environment: I. Analysis of the water extraction pattern. Field Crops Research 33, 81–97.
| Water extraction by grain sorghum in a sub-humid environment: I. Analysis of the water extraction pattern.Crossref | GoogleScholarGoogle Scholar |
Sadok W (2013) Root-based hydraulic restriction as a basis for drought tolerance in wheat. In ‘Proceedings of InterDrought-IV.’ ‘Proceedings of Interdrought IV, Perth’. (Eds R. Tuberosa, N. Turner, M. Cakir) p. 81. (EECW: Perth)
Sanchez AC, Subudhi PK, Rosenow DT, Nguyen HT (2002) Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor (L.) Moench). Plant Molecular Biology 48, 713–726.
| Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor (L.) Moench).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjsFWrt7o%3D&md5=be3a7a351e6a679b0a646f26fc90f0ebCAS | 11999845PubMed |
Sinclair TR, Hammer GL, van Oosterom EJ (2005) Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Functional Plant Biology 32, 945–952.
| Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Messina CD, Beatty A, Samples M (2010) Assessment across the United States of the benefits of altered soybean drought traits. Agronomy Journal 102, 475–482.
| Assessment across the United States of the benefits of altered soybean drought traits.Crossref | GoogleScholarGoogle Scholar |
Subudhi PK, Rosenow DT, Nguyen HT (2000) Quantitative trait loci for the stay-green trait in sorghum (Sorghum bicolor L. Moench): consistency across genetic backgrounds and environments. Theoretical Applied Genetics 101, 733–741.
| Quantitative trait loci for the stay-green trait in sorghum (Sorghum bicolor L. Moench): consistency across genetic backgrounds and environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXosFyhurw%3D&md5=0b4f47726115d819b71e7e7743f4f6ddCAS |
Tao YZ, Henzell RG, Jordan DR, McIntyre CL (2000) Identification of genomic regions associated with stay-green in sorghum by testing RILs in multiple environments. Theoretical and Applied Genetics 100, 1225–1232.
| Identification of genomic regions associated with stay-green in sorghum by testing RILs in multiple environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlsF2gsbs%3D&md5=e0fc5253b8e6201d9cd030f21c8902a7CAS |
Tardieu F (2012) Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. Journal of Experimental Botany 63, 25–31.
| Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1yms73L&md5=12b81cc70f5f1e9825b32c15df6fdd4cCAS | 21963615PubMed |
Thomas H, Howarth CJ (2000) Five ways to stay green. Journal of Experimental Botany 51, 329–337.
| Five ways to stay green.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhsVKqurc%3D&md5=19d3cfe284e44795cd34391bcad93589CAS | 10938840PubMed |
Tuinstra MR, Grote EM, Goldsbrough PB, Ejeta G (1996) Identification of quantitative trait loci associated with pre-flowering drought tolerance in sorghum. Crop Science 36, 1337–1344.
| Identification of quantitative trait loci associated with pre-flowering drought tolerance in sorghum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmsFCkur0%3D&md5=5d1fd52d262d4629db027d01c3f06353CAS |
Tuinstra MR, Grote EM, Goldsbrough PB, Ejeta G (1997) Genetic analysis of post-flowering drought tolerance and components of grain development of Sorghum bicolor (L.) Moench. Molecular Breeding 3, 439–448.
| Genetic analysis of post-flowering drought tolerance and components of grain development of Sorghum bicolor (L.) Moench.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXit1Kit7c%3D&md5=1a19cc80cf05350c66a89b69529430bcCAS |
Tuinstra MR, Ejeta G, Goldsbrough P (1998) Evaluation of near isogenic sorghum lines contrasting for QTL markers associated with drought tolerance. Crop Science 38, 835–842.
| Evaluation of near isogenic sorghum lines contrasting for QTL markers associated with drought tolerance.Crossref | GoogleScholarGoogle Scholar |
Vadez V, Deshpande SP, Kholová J, Hammer GL, Borrell AK, Talwar HS, Hash CT (2011) Stay-green quantitative trait loci’s effects on water extraction, transpiration efficiency and seed yield depend on recipient parent background. Functional Plant Biology 38, 553–566.
| Stay-green quantitative trait loci’s effects on water extraction, transpiration efficiency and seed yield depend on recipient parent background.Crossref | GoogleScholarGoogle Scholar |
Vadez V, Kholová J, Medina S, Kakkera A, Anderberg H (2014) Transpiration efficiency: new insight on an old story. Journal of Experimental Botany
| Transpiration efficiency: new insight on an old story.Crossref | GoogleScholarGoogle Scholar | 24600020PubMed |
van Oosterom EJ, Carberry PS, O’Leary GJ (2001) Simulating growth, development, and yield of tillering pearl millet. I. Leaf area profiles on main shoots and tillers. Field Crops Research 72, 51–66.
| Simulating growth, development, and yield of tillering pearl millet. I. Leaf area profiles on main shoots and tillers.Crossref | GoogleScholarGoogle Scholar |
van Oosterom EJ, Borrell AK, Chapman SC, Broad IJ, Hammer GL (2010a) Functional dynamics of the nitrogen balance of sorghum. I. N demand of vegetative plant parts. Field Crops Research 115, 19–28.
| Functional dynamics of the nitrogen balance of sorghum. I. N demand of vegetative plant parts.Crossref | GoogleScholarGoogle Scholar |
van Oosterom EJ, Chapman SC, Borrell AK, Broad IJ, Hammer GL (2010b) Functional dynamics of the nitrogen balance of sorghum. II. Grain filling period. Field Crops Research 115, 29–38.
| Functional dynamics of the nitrogen balance of sorghum. II. Grain filling period.Crossref | GoogleScholarGoogle Scholar |
van Oosterom E, Borrel AK, Deifel KS, Hammer GL (2011) Does increased leaf appearance rate enhance adaptation to post anthesis drought stress in sorghum? Crop Science 51, 2728–2740.
| Does increased leaf appearance rate enhance adaptation to post anthesis drought stress in sorghum?Crossref | GoogleScholarGoogle Scholar |
Welcker C, Boussuge B, Bencivenni C, Ribaut JM, Tardieu F (2007) Are source and sink strengths genetically linked in maize plantssubjected to water deficit? A QTL study of the responses of leaf growth and of anthesis–silking interval to water deficit. Journal of Experimental Botany 58, 339–349.
| Are source and sink strengths genetically linked in maize plantssubjected to water deficit? A QTL study of the responses of leaf growth and of anthesis–silking interval to water deficit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlOltL8%3D&md5=535ea0f36bcaef099820a9620e690adbCAS | 17130185PubMed |
Xu W, Subudhi PK, Crasta OR, Rosenow DT, Mullet JE, Nguyen HT (2000) Molecular mapping of QTLs conferring stay-green in grain sorghum (Sorghum bicolor L.Moench). Genome 43, 461–469.
| Molecular mapping of QTLs conferring stay-green in grain sorghum (Sorghum bicolor L.Moench).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXksVGgs7s%3D&md5=691460caa70692b55b133acee735b7a6CAS | 10902709PubMed |