Novel chlorophylls and new directions in photosynthesis research
Yaqiong Li A and Min Chen A BA School of Biological Sciences, The University of Sydney, NSW 2006, Australia.
B Corresponding author. Email: min.chen@sydney.edu.au
This review originates from the Peter Goldacre Award 2013 of the Australian Society of Plant Scientists that was received by the second author.
Functional Plant Biology 42(6) 493-501 https://doi.org/10.1071/FP14350
Submitted: 12 December 2014 Accepted: 6 February 2015 Published: 6 March 2015
Abstract
Chlorophyll d and chlorophyll f are red-shifted chlorophylls, because their Qy absorption bands are significantly red-shifted compared with chlorophyll a. The red-shifted chlorophylls broaden the light absorption region further into far red light. The presence of red-shifted chlorophylls in photosynthetic systems has opened up new possibilities of research on photosystem energetics and challenged the unique status of chlorophyll a in oxygenic photosynthesis. In this review, we report on the chemistry and function of red-shifted chlorophylls in photosynthesis and summarise the unique adaptations that have allowed the proliferation of chlorophyll d- and chlorophyll f-containing organisms in diverse ecological niches around the world.
Additional keywords: Acaryochloris, chlorophyll d, chlorophyll f, cyanobacteria, hongdechloris, photosynthesis.
General distributions and properties of various chlorophylls
Chlorophyll (Chl) is derived from the Greek words meaning green (chloros) and leaf (phyllon) (Pelletier and Caventou 1818). They are greenish photopigments comprising a chlorin ring and a magnesium atom (Mg) with a long hydrophobic phytol chain attached (Fig. 1). Chlorophylls are only found in oxygenic photosynthetic organisms and they play a vital role in light-harvesting and energy transduction (Blankenship 2014). So far, five chlorophylls – named Chl a, b, c, d and f after the order of their discoveries – have been found in oxygenic photosynthetic organisms, including plants, algae and cyanobacteria (Chen et al. 2010; Chen 2014a, 2014b).
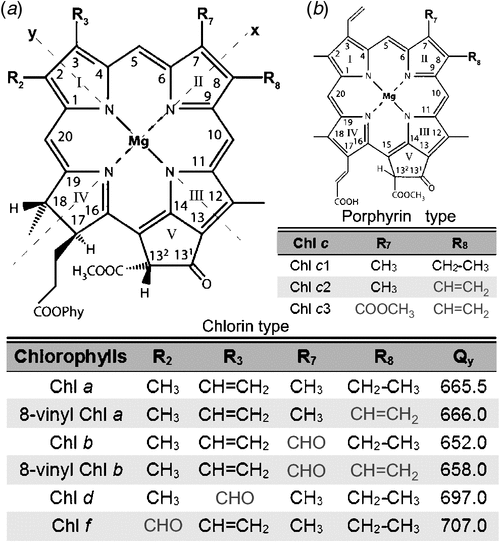
|
Chl a functions as a primary electron donor in nearly all oxygenic photosynthetic organisms (Björn et al. 2009), with a remarkable exception being found in the cyanobacterial group Acaryochloris marina (Miyashita et al. 1996). Chl b is found in the majority of eukaryotic photosynthetic organisms (virtually all plants and green algae), and it is considered to be the primary accessory pigment for light harvesting and energy transfer. Additionally, Chl b is present also in prochlorophytes, a group of cyanobacteria (La Roche et al. 1996). Chl c is a common name for more than three closely related pigments named Chl c1, Chl c2, and so on (Zapata et al. 2006). Structurally, they are quite different from other chlorophylls, and are porphyrins instead of chlorins (Fig. 1). They function as accessory photopigments in light-harvesting complexes and are universally present in many groups of marine algae, such as diatoms, brown algae and dinoflagellates (Zapata et al. 2006). Chl d was first discovered as a minor component in red algae pigment extractions 70 years ago (Manning and Strain 1943). Later, it was considered to be an artificial by-product created during the extraction process (Holt and Morley 1959; Holt 1961). The question of the presence of Chl d in photosynthetic organisms naturally was not resolved until the discovery of A. marina in 1996. Chl d is the predominant chlorophyll in this cyanobacterial species, constituting over 95% of total chlorophylls, depending on the culture conditions (Miyashita et al. 1996). Chl d can replace nearly all of the functions of Chl a in A. marina, not only in light-harvesting complexes (Chen et al. 2002; Tomo et al. 2011), but also in reaction centres (Hu et al. 1998; Chen et al. 2005; Tomo et al. 2007). So far, several strains of Chl d-containing organisms have been isolated and cultured, and all of those belong to one genus of cyanobacteria, A. marina (Miyashita et al. 1996; Murakami et al. 2004; Miller et al. 2005; Mohr et al. 2010; Larkum et al. 2012). There is very little information regarding Chl e due to only two cases being mentioned as, unpublished data around the 1940s (Chen et al. 2010). All referenced work regarding Chl e was HH Strain’s, unpublished data in 1943 (pigment extraction from Tribonema bombycinum) and 1948 (pigment extraction from Vaucheria hamata) (Chen et al. 2010). Additionally, Chl e has never been isolated and chemically characterised, therefore, the existence of Chl e still needs to be proved. To avoid the subsequent confusion, the newly discovered chlorophyll as ‘chlorophyll f’ based on the order of chlorophylls reported. Chl f is the most red-shifted chlorophyll found to date (Chen et al. 2010). It was first discovered in a methanolic pigment extraction of stromatolites collected from Shark Bay, Hamelin Pool, Western Australia (Chen et al. 2010). Samples were cultured under infrared light (720 nm LED light) for the initial purpose of isolating new Chl d-containing organisms. Further studies indicated that Chl f only occupied around 10% of the total chlorophylls in the marine filamentous cyanobacterium named Halomicronema hongdechloris, which was isolated and purified from the stromatolite sample as above. Further, Chl f is the first chlorophyll known in which its biosynthesis is directly induced by infrared light (Chen et al. 2012). More recently, Chl f was reported in other cyanobacteria (Akutsu et al. 2011; Airs et al. 2014; Gan et al. 2014; Miyashita et al. 2014).
Spectral and photochemical properties of chlorophylls
The absorption spectra of chlorophylls can be described according to the ‘four orbital’ model (Gouterman 1961). Absorption spectra of chlorophyll show the electronic transitions along the x-axis of the chlorophyll running through the two nitrogen (N) atoms of rings II and IV, and along the y-axis through the N atoms of rings I and III (Fig. 1). The two main absorption bands in the blue and red regions are called Soret and Q bands, respectively, and these arise from π→π* transitions of four frontier orbitals (Weiss 1978; Petke et al. 1979; Hanson 1991). The lack of absorbance in the green spectral region, the so-called the ‘green window’, is responsible for the green colours of chlorophylls. The two lowest-energy transitions are called Q bands and the two highest-energy transitions are named B bands, also commonly called ‘Soret bands’ (Blankenship 2014). Taking Chl a as an example, the spectrum is characterised by two roughly separated Soret (B) bands at ~444 and 392 nm and a relatively strong Qy band near 667 nm in 100% methanol at 183 K (Li et al. 2013).
Structurally, Chls b, d and f are identical to Chl a with a chlorin macrocycle and a long phytol isoprenoid chain attached at C17, except for the substitution of the (formyl group at different positions of the chlorin macrocycle (Fig. 1). Chl f and b share the same molecular formula (C55H70N4O6Mg), but the substitution of the formyl group is at the C2 or C7 position respectively (Fig. 1). Chl d (C54H68N4O6Mg) possesses a formyl group at the C3 position, whereas a vinyl group is found in Chl a at this position (Fig. 1). These differences of macrocycle peripheral groups significantly affect the absorption spectra of the chlorophylls (Hoober et al. 2007). Compared with Chl a, the Soret band of Chl b is red-shifted to 457 nm compared with 435 nm of Chl a and its Qy band is blue-shifted to 646 nm in 100% acetone (Jeffrey and Humphrey 1975). The main Soret and Qy bands of Chl d are red-shifted to 470 and 700 nm in 100% methanol at 183K respectively (Li et al. 2013). The main Soret band of Chl f is blue-shifted to 408 nm and its Qy band is red-shifted to 712 nm in 100% methanol at 183K (Li et al. 2013), so that Chl f has the widest ‘green window’ among all known chlorophylls (Fig. 2) (Chen and Scheer 2013). Both Chl d and Chl f have the red-shifted Qy peaks compared with that of Chl a, therefore, Chl d and Chl f are also named as ‘red-shifted’ chlorophylls. Photosynthetic organisms containing red-shifted chlorophylls can thrive in environments where the infrared light is enriched and visible light is limited (Kühl et al. 2005), whereas the Chl a- photosynthesis is limited by available light due to its absorption properties (Fig. 2). In contrast, Chl b, a blue-shifted chlorophyll that extends the absorption of light towards the blue side of the ‘green window’, is more adapted to terrestrial light environments (Chen and Scheer 2013).
Cyanobacterial strains possess unique chlorophylls
Prochlorococcus spp. are the smallest photosynthetic organisms known to date with the spherical diameter of 0.5 to 0.7 µm (Partensky et al. 1999). They use 8-vinyl Chl a and 8-vinyl Chl b (also named as 3,8, divinyl-Chl a and 3,8, divinyl-Chl b) instead of using the Chl a and Chl b (having monovinyl at C3 position, Fig. 1) in their photosynthetic system (Goericke and Repeta 1992; Chisholm et al. 1992; Partensky et al. 1993, 1999). The Soret band of both 8-vinyl Chl a and 8-vinyl Chl b are red-shifted by 8–10 nm, compared with Chl a and Chl b, extending their absorbance at the blue side of the ‘green window’ (Morel et al. 1993; Moore et al. 1995; Partensky et al. 1999). The presence of 8-vinyl Chl a and 8-vinyl Chl b allows Prochlorococcus spp. to adapt to the ecological niches in the open-ocean, where blue light wavelengths are enriched (Partensky et al. 1999; Ito and Tanaka 2011).
Acaryochloris marina is a unicellular cyanobacterium that uses Chl d (constituting up to 90–99% of total chlorophylls) as its major photopigment to carry out oxygenic photosynthesis (Miyashita et al. 1996; Mimuro et al. 2004; Lin et al. 2013). A. marina strains are found widely through various ecological systems (Loughlin et al. 2013). Up to date, several strains of A. marina have been isolated and cultured, A. marina MBIC11017 (Miyashita et al. 1996), Acaryochloris sp. AWAJI-1 (Murakami et al. 2004), Acaryochloris sp. CCMEE 5410 (Miller et al. 2005), Acaryochloris sp. HICR111A (Mohr et al. 2010), and Acaryochloris sp. MPGRS1 (Larkum et al. 2012). Chl dʹ and Pheo a are present as minor components, but neither Chl aʹ nor Pheo d is found in A. marina (Akiyama et al. 2001). Acaryochloris sp. MBIC 11017 has a unique phycobiliprotein arrangement, a rod-array structure rather than a typical phycobilisome (PBS) (Marquardt et al. 1997; Hu et al. 1998; Chen et al. 2009). Each rod consists of four discs that are formed by three hexamers (α6β6). This structure is located at the stromal side of the thylakoid membrane and attached primarily to the PSII-antenna supercomplexes (Chen et al. 2009). Alpha-carotene and its derivatives are only found in two genera of cyanobacteria among all the prokaryotes: Prochlorococcus spp. and Acaryochloris spp. In Acaryochloris, α-carotene carries out the same function as β-carotene in the other cyanobacteria (Loughlin et al. 2013).
There are four different species of Chl f-containing cyanobacteria reported to date, H. hongdechloris, cyanobacterium strain KC1, Leptolyngbya sp. JSC-1 and Chlorogloeopsis fritschii PCC 6912 (Akutsu et al. 2011; Airs et al. 2014; Gan et al. 2014). H. hongdechloris belongs to the genus Halomicronema based on phylogenetic analysis and morphological features (Chen et al. 2012). It is reported that H. hongdechloris contains four main carotenoids and two chlorophylls, Chl a and f (Chen et al. 2012). Chl a is predominant chlorophyll under different light conditions (Chen et al. 2012; Li et al. 2014). Therefore, H. hongdechloris can acclimatise its pigment profiles to meet the requirements of the light environment: using Chl f to absorb infrared light under infrared-light-conditions and using phycobiliproteins and Chl a to absorb the visible light region of 400 to 700 nm. This infrared-light-inducible synthesis of Chl f appears to be the case for the other recent discoveries of Chl f-containing organisms: cyanobacterium strain KC1 (Akutsu et al. 2011), Leptolyngbya sp. JSC-1 (Gan et al. 2014) and Chlorogloeopsis fritschii PCC 6912 (Airs et al. 2014). Cyanobacterium strain KC1 is a unicellular cyanobacterium; it is closely related to unicellular cyanobacteria Aphanocapsa muscicola and has a sister relationship to clade of Acaryochloris spp. (Akutsu et al. 2011; Miyashita et al. 2014). The sequence of 16s rDNA between strains KC1 and H. hongdechloris only have 92% similarity (Miyashita et al. 2014). Similar to H. hongdechloris, the biosynthesis of Chl f only occurs when infrared light is present and Chl a always functions as major photopigment, under various light conditions. Therefore, it was suggested that Chl f may function not as primary donor in reaction centres, but instead as an antenna component where an uphill energy transfer would be required to deliver the excitation energy from Chl f to Chl a in reaction centres (Chen and Blankenship 2011).
Leptolyngbya sp. JSC-1 was isolated from a floating cyanobacterial mat from hot springs (~45°C) in the Yellowstone National Park, Montana, USA (Brown et al. 2010; Gan et al. 2014). Since Leptolyngbya sp. JSC-1 can thrive in hot environments up to 60°C; it is classified as a thermotolerant cyanobacterium. Leptolyngbya sp. JSC-1 is filamentous with two morphotypes: isometric cells (length of 2.52 ± 0.41 µm, width of 2.16 ± 0.13 µm), and elongated cylindrical cells (length of 2.89 ± 0.27 µm, width of 1.62 ± 0.17 µm) (Brown et al. 2010). The cell sizes of cyanobacterium strain KC1 (unicellular cells with diameter of 1.3–2.0 µm and length of 1.3–3.0 µm) and Leptolyngbya sp. JSC-1 are much bigger than those of H. hongdechloris (length of 1.0–1.3 µm, width of 0.6–0.8 µm) (Chen et al. 2012; Miyashita et al. 2014). Leptolyngbya JSC-1 is a representative of a new genus of Leptolyngbya closed to Leptolyngbya frigida Ant.LH70.1 and Leptolyngbya sp. CENA 103 with <95% similarity (Fig. 3; Brown et al. 2010). It has been reported that strain Leptolyngbya sp. JSC-1 has a unique capability of synthesising nine carotenoids and three chlorophylls (Chl a, Chl d and Chl f) in response to different light conditions (Brown et al. 2010; Gan et al. 2014).

|
Chlorogloeopsis fritschii PCC 6912 (C. fritschii) was first isolated from the soils of paddy fields in India (Mitra 1950). C. fritschii has a diverse morphology and diversity of function depending on growth conditions (Evans et al. 1976), such as filaments and aseriate forms of irregular clumps of cells. Aseriate cells dominate under light condition of infrared light and natural light (Airs et al. 2014). C. fritschii is able to synthesise six carotenoids and three chlorophylls (Chl a, Chl d and Chl f) (Airs et al. 2014). We note that the production of Chl f and Chl d are found in C. fritschii grown under both infrared light and natural light conditions (Airs et al. 2014). The content of Chl f was maximised to ~6% of that total chlorophylls under infrared light condition (Airs et al. 2014), only half of that observed in H. hongdechloris grown under same condition. The highest ratio of Chl d to Chl a was maximum of 1% observed in infrared light-grown cells (Airs et al. 2014). Further, the ratio of Chl f to Chl d remained relatively unchanged when cells grown under both infrared light and nature light (Airs et al. 2014). In addition, the aseriate forms of C. frischii cells may create a microenvironment with enriched infrared light, due to the self-shading (Airs et al. 2014).
Predicting the occurrence of Chl f-containing organisms and towards the isolation of novel Chl f-containing organisms
H. hongdechloris was isolated from the inner layers of stromatolites collected from Shark Bay, Western Australia (Chen et al. 2010, 2012). Since Chl f was discovered, only three other studies have reported the occurrence of Chl f-containing organisms. A comparison of the four organisms that have Chl f demonstrates that these organisms thrive in quite different ecological niches, including freshwater lakes, hot springs, the ocean and soil. These organisms also have very distinct morphological features. Two are filamentous and one is unicellular and all belong to different phylogenetic groups (Fig. 3). However, one common feature shared among them is the presence of Chl f when they are cultured under infrared light conditions. Thus, using >700 nm LEDs could play a significant role discovering the presence of Chl f and help improve our understanding of the ecological distribution of Chl f-containing organisms. This presents the possibility that Chl f production might be overlooked in many culture collections because infrared light not commonly applied in the culture of cyanobacteria or algae. The variation of Chl f-containing cyanobacteria also supports the idea that Chl f is the result of environmental adaptation.
Such organisms should only be found in certain habitats that are enriched in far-red light and depleted in visible light; for example, the interior of the microbial mat within the stromatolite where the first Chl f-containing organism was discovered (Chen et al. 2010, 2012). The visible region of light is absorbed by the photosynthetic organisms harboured in the upper layer. Cells residing beneath this layer are capable of utilising Chl f (or Chl d) in order to capture leftover infrared light to drive photosynthesis. However, knowledge of the distributions of Chl f-containing organisms in the environment is still largely unknown.
The acquisition of new or additional chlorophylls by photosynthetic organisms is thought to be an adaptation to the light quality of their niches (Croce and van Amerongen 2014). An organism that only contains Chl a (e.g. Synechococcus) cannot survive in an environment with 720 nm light (Duxbury et al. 2009), whereas H. hongdechloris thrives in infrared light (730 nm LED). Different O2 evolution activities were observed between Chl f-containing infrared light-grown cells and white-light-grown cells, when illuminated by infrared light, which confirms the spectral expansion of oxygenic photosynthesis afforded by the presence of Chl f in H. hongdechloris (Li et al. 2014). These results not only demonstrate the benefit of possessing Chl f of extending the range of PAR to the infrared region, but also indicate that Chl f must contribute to the energy input of those cyanobacteria under such unique light conditions. Thus, the study of Chl f could improve our understanding of the ecological significance of spectral extension in natural photosynthetic systems (Chen and Blankenship 2011).
Oxygenic photosynthesis and its physical limits
Photosynthesis is a biological solar energy storage process with two photo-excitation photosystem/reaction centres in oxygenic photosynthesis (Blankenship and Hartman 1998; Barber 2009; Kalyanasundaram and Graetzel 2010). The electron-donors P700 (PSI) and P680 (PSII), both Chl a molecules, will elevate the redox spans that allow the charge separation in the reaction centre and transfer the electron from water to NADP thermodynamically downhill (Fig. 4).
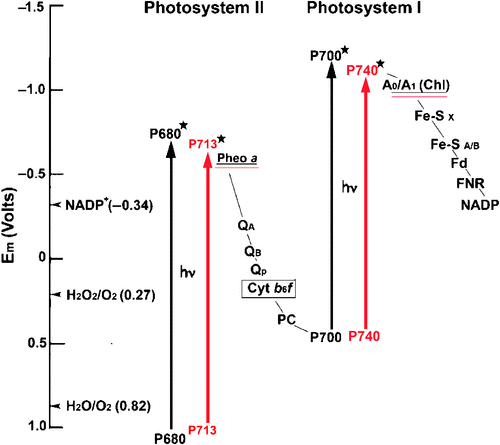
|
In general, oxygenic photosynthesis driven by Chl a and Chl b (not red-shifted chlorophylls) has long wavelength absorbance spectra extending to limits of ~700 nm due to the high energy requirements of splitting water and oxygen production (Björn et al. 2009). Removal of electrons from water requires powerful oxidative potential and hence the presence of P680 (Em = 1.23 V) is vital for oxygenic photosynthesis (Dau and Haumann 2008), although the long wavelength light has an excited state redox potential that is sufficiently negative to power the reduction of the primary electron acceptor, such as anoxygenic (non-oxygen evolving) photosynthesis driven by bacteriochlorophylls (Blankenship and Prince 1985).
Chl a was thought to be the only chlorophyll that can generate enough energy to split water and evolve oxygen as a by-product of oxygenic photosynthesis (Björn et al. 2009). It was thought that long wavelength light (>700 nm) contains low energy photons and is not sufficiently energetic to oxidise water (Blankenship and Prince 1985; Chen and Blankenship 2011). The traditional view of oxygenic photosynthesis was challenged with the discovery of red-shifted chlorophylls. Since 1996, research on Chl d-driven photosynthesis has expanded our understanding of the molecular mechanism of oxygenic photosynthesis (Loughlin et al. 2013). The investigation of Chl d and its photosynthetic reactions in A. marina has overturned the long-standing belief concerning the ‘red-edge’ of photosynthesis driven by Chl a.
The discovery of Chl f and its containing organisms open the PAR window even wider, predicting to extend the photosynthetic absorbance region up to 760 nm, far beyond the absorbance limits of 700 nm for Chl a and 740 nm for Chl d.
Previous studies have demonstrated that ‘red chlorophylls’ with Qy absorption maxima up to 760 nm can be found in several oxygenic photosynthetic organisms (Koehne et al. 1999; Schlodder et al. 2005; Wilhelm and Jakob 2006). These ‘red chlorophylls’ are mostly found in light-harvesting complexes, although the formation and function of those ‘red chlorophylls’ in energy storage is under debate (Melkozernov and Blankenship 2003; Corbet et al. 2007). The properties of these ‘red chlorophylls’ are accomplished using Chl a, whereby its spectrometric properties are modified by the protein environment, rather than modifying the chemical structure of the chlorophyll. However, the red-shifted spectrometric properties of Chl d and Chl f are accomplished by the modification of the chemical structure of the chlorophyll (Chen et al. 2010; Willows et al. 2013).
Chen and Blankenship (2011) suggested that an uphill energy transfer is needed to deliver excitation derived from red-shifted photons to the Chl a-containing reaction centres which had the normal absorbance spectra (P680 and P700) that are significantly ‘bluer’ (i.e. higher energy) than the red-shifted chlorophylls. In the Chl d-containing cyanobacterium, A. marina, Chl d (P740) replaces Chl a in P700, which prevents the energy transfer from a significant uphill energy transfer (Hu et al. 1998; Mimuro et al. 2000; Chen and Blankenship 2011). However, the red-shifted chlorophylls might encounter the energy losses during the subsequent photochemistry that critically affect the efficiency of electron-transfer, and thereby energy storage. A recent study investigating the redox potential of the Chl d special pairs in A. marina reported no differences for the redox potential level between Chl a-containing cyanobacteria and A. marina (Allakhverdiev et al. 2010; Allakhverdiev et al. 2011). For PSII reaction centre in A. marina, there is general agreement that Chl a is replaced by Chl d, at least at accessory sites (ChlD1 and ChlD2) (Chen et al. 2005; Tomo et al. 2007) and Pheo a (instead of Pheo d) is the primary acceptor of D1-side as in other oygenic photosynthetic organisms (Tomo et al. 2007, 2008). However, a controversy over the identities of the special pair chlorophylls in RC II has been debated more than 10 years due to the lack of the purified PSII reaction centre complexes (Chen et al. 2005; Itoh et al. 2007; Tomo et al. 2007). Further investigation is required for understanding the molecular mechanism of Chl d-photosynthesis, including the nature of RCII in A. marina. The energy transfer efficiency analysis of Chl d-photosynthesis revealed similar rates between Chl a-photosynthtic systems and Chl d-photosynthetic systems (Mielke et al. 2013).
Whether Chl f is involved in charge separation in the reaction centres, or only captures light energy in light harvesting complex, is still unknown. If Chl f is functional in light harvesting complexes, this means an uphill energy transfer is required for Chl f to deliver the energy to the Chl a in reaction centres. A new energy transfer pathway may be expected to support such a theory. However, if Chl f is involved in charge separation in the reaction centre, it is also unclear whether Chl f is capable of using the lower energy to oxidise water and produce oxygen. Water oxidation and oxygen production, driven by photosynthesis, requires higher energy input than non-oxygenic photosynthesis and it is this that determines the minimum energy input for photosynthetic reactions.
Chen and Blankenship (2011) point out the importance of the spectral region >700 nm. Due to the maximum solar spectrum occurring in spectral region >700 nm, and when the solar spectrum is represented as photon flux, every increment in the ability to utilise photons >700 nm can have a significant effect on the available energy utilised for photosynthesis. The ability to utilise light 50 nm outside the PAR spectrum (to 750 nm) results in an increase in the number photons available for photosynthesis by 19%. In oxygenic photosynthetic organisms, this expansion of the solar spectrum can be accomplished using available pigments such as red-shifted chlorophylls, especially the most red-shifted chlorophyll to date, Chl f. Such an expansion is recognised as a good potential source of photons to drive photosynthesis at higher efficiency.
The discovery of red-shifted chlorophylls has forced a re-evaluation of our understanding of the minimum threshold energy required for oxygenic photosynthesis. Studies of red-shifted chlorophylls have contributed to a better understanding of the molecular mechanisms of photosynthesis driven by red-shifted chlorophylls. The question remains as to whether or not this limit can be extended even further to longer wavelengths, and if so, how far the physical limit of oxygenic photosynthesis can hold. Understanding the mechanisms underlying the putative functions of Chl f in the reaction centres could help us to understand what the minimum threshold energy for oxygenic photosynthesis is. Chl f could possibly contribute to improving the efficiency of photosynthesis by extending the photosynthetically available spectrum further into the infrared than previously thought.
There are two potential applications of red-shifted chlorophylls. First, such chlorophylls would increase the ability of photosynthetic organisms to use light in an additional region of the solar spectrum. This could lead to significant improvements in agricultural efficiency or bioenergy storage if red-shifted chlorophylls can be integrated into algae or higher plants. This would theoretically give Chl a, b or c-containing oxygenic photosynthetic organisms access to approximately an additional 19% photon flux compared with other oxygenic photosynthetic organisms, which can only can absorb standard PAR (Chen and Blankenship 2011). An additional 19% photon flux in any oxygenic photosynthetic organism could be very significant on a global bioenergy scale. Such an increase could significantly increase food, fuel, or biomass production. Second, red-shifted chlorophylls may also be useful for remote sensing and detection of plants that contain this unique pigment due to their distinguishing red-shifted fluorescent properties.
Acknowledgements
A special thank you to the Australian Society of Plant Scientists and Functional Plant Biology for their encouragement through the Goldacre award to MC (2013). MC also thanks the Australian Research Council for financial supports through the ARC Centre of Excellence for Translational Photosynthesis (CE1400015), Discovery Project (DP120100286) and ARC Future Fellow (FT120100464). YL thanks support from the China Scholarship Council.
References
Airs RL, Temperton B, Sambles C, Farnham G, Skill SC, Llewellyn CA (2014) Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and near-infrared radiation. FEBS Letters 588, 3770–3777.| Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and near-infrared radiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVKnu7%2FP&md5=d71bda08af205ff3541b437d5d4fab63CAS | 25176411PubMed |
Akiyama M, Miyashita H, Kise H, Watanabe T, Miyachi S, Kobayashi M (2001) Detection of chlorophyll d and pheophytin a in a chlorophyll d-dominating oxygenic photosynthetic prokaryote Acaryochloris marina. Analytical Sciences 17, 205–208.
| Detection of chlorophyll d and pheophytin a in a chlorophyll d-dominating oxygenic photosynthetic prokaryote Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD383lsVKmtw%3D%3D&md5=0df87d53603fafecbfdb30e23abe9212CAS | 11993664PubMed |
Akutsu S, Fujinuma D, Furukawa H, Watanabe T, Ohnishi-Kameyama M, Ono H, Ohkubo S, Miyashita H, Kobayashi MC (2011) Pigment analysis of a chlorophyll f-containing cyanobacterium strain KC1 isolated from Lake Biwa. Photomedicine and Photobiology 33, 35–40.
Allakhverdiev SI, Tomo T, Shimada Y, Kindo H, Nagao R, Klimov VV, Mimuro M (2010) Redox potential of pheophytin a in photosystem II of two cyanobacteria having the different special pair chlorophylls. Proceedings of the National Academy of Sciences of the United States of America 107, 3924–3929.
| Redox potential of pheophytin a in photosystem II of two cyanobacteria having the different special pair chlorophylls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtFylsL8%3D&md5=d9f05fe6d36d7142ff7259155a36bb62CAS | 20142495PubMed |
Allakhverdiev SI, Tsuchiya T, Watabe K, Kojima A, Los DA, Tomo T, Klimov VV, Mimuro M (2011) Redox potentials of primary electron acceptor quinone molecule (QA) - and conserved energetics of photosystem II in cyanobacteria with chlorophyll a and chlorophyll d. Proceedings of the National Academy of Sciences of the United States of America 108, 8054–8058.
| Redox potentials of primary electron acceptor quinone molecule (QA) - and conserved energetics of photosystem II in cyanobacteria with chlorophyll a and chlorophyll d.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmsVSrsr8%3D&md5=bcfa7045c9a273b25ea808ce61745ee1CAS | 21521792PubMed |
Barber J (2009) Photosynthetic energy conversion: natural and artificial. Chemical Society Reviews 38, 185–196.
| Photosynthetic energy conversion: natural and artificial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFWjtL3F&md5=db9081cb32d895b0efe44dcf47cee297CAS | 19088973PubMed |
Björn L, Papageorgiou G, Blankenship R (2009) A viewpoint: why chlorophyll a? Photosynthesis Research 99, 85–98.
| A viewpoint: why chlorophyll a? Crossref | GoogleScholarGoogle Scholar | 19125349PubMed |
Blankenship RE (2014) Photosynthetic pigments: structure and spectroscopy. In ‘Molecular mechanisms of photosynthesis’. (Ed. RE Blankenship) pp. 41−57. (John Wiley & Sons: Oxford)
Blankenship RE, Hartman H (1998) The origin and evolution of oxygenic photosynthesis. Trends in Biochemical Sciences 23, 94–97.
| The origin and evolution of oxygenic photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisVOms74%3D&md5=963f7c55a579c36612640da1ac1bc2c1CAS | 9581499PubMed |
Blankenship RE, Prince RC (1985) Excited-state redox potentials and the Z scheme of photosynthesis. Trends in Biochemical Sciences 10, 382–383.
| Excited-state redox potentials and the Z scheme of photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XjtlKjtQ%3D%3D&md5=0fdb26f79f828b7c621da7eeee599bd6CAS |
Brown II, Bryant DA, Casamatta D, Thomas-Keprta KL, Sarkisova SA, Shen G, Graham JE, Boyd ES, Peters JW, Garrison DH, McKay DS (2010) Polyphasic characterization of a thermotolerant siderophilic filamentous cyanobacterium that produces intracellular iron deposits. Applied and Environmental Microbiology 76, 6664–6672.
| Polyphasic characterization of a thermotolerant siderophilic filamentous cyanobacterium that produces intracellular iron deposits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlartr%2FK&md5=c7449a7ed22690ba341bb3232bd9083fCAS | 20709851PubMed |
Chen M (2014a) ‘Chlorophylls and photosynthesis. Case Study 1.2, Plants in action.’ (Australian Society of Plant Scientists) Available at: http://plantsinaction.science.uq.edu.au
Chen M (2014b) Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annual Review of Biochemistry 83, 317–340.
| Chlorophyll modifications and their spectral extension in oxygenic photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFOhtrjI&md5=beb9d06ebd26ece241132e125ac52e4dCAS | 24635479PubMed |
Chen M, Blankenship RE (2011) Expanding the solar spectrum used by photosynthesis. Trends in Plant Science 16, 427–431.
| Expanding the solar spectrum used by photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpvFWqsr0%3D&md5=adaea76883df5e5352fe1911365490f8CAS | 21493120PubMed |
Chen M, Scheer H (2013) Extending the limits of natural photosynthesis and implication for technical light harvesting. Journal of Porphyrins and Phthalocyanines 17, 1–15.
| Extending the limits of natural photosynthesis and implication for technical light harvesting.Crossref | GoogleScholarGoogle Scholar |
Chen M, Quinnell RG, Larkum AWD (2002) The major light harvesting pigment protein of Acaryochloris marina. FEBS Letters 514, 149–152.
| The major light harvesting pigment protein of Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xis1ejtrw%3D&md5=de743724bd7e0e8f044478ba2e625b7fCAS | 11943141PubMed |
Chen M, Telfer A, Lin S, Pascal A, Larkum AWD, Barber J, Blankenship RE (2005) The nature of the photosystem II reaction centre in the chlorophyll d-containing prokaryote, Acaryochloris marina. Photochemical & Photobiological Sciences 4, 1060–1064.
| The nature of the photosystem II reaction centre in the chlorophyll d-containing prokaryote, Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Cit77M&md5=214aaef244f1ccb55536c6cab95ce016CAS |
Chen M, Floetenmeyer M, Bibby T (2009) Supramolecular organization of phycobiliproteins in the chlorophyll d-containing cyanobacterium Acaryochloris marina. FEBS Letters 583, 2535–2539.
| Supramolecular organization of phycobiliproteins in the chlorophyll d-containing cyanobacterium Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptlahu7g%3D&md5=c0ad2ce7b099e53dd6fc0ff242d71dbfCAS | 19596002PubMed |
Chen M, Schliep M, Willows RD, Cai Z-L, Neilan BA, Scheer H (2010) A red-shifted chlorophyll. Science 329, 1318–1319.
| A red-shifted chlorophyll.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFajs7rM&md5=17fe33c88a9cec88d5b9d7fe75f3f822CAS | 20724585PubMed |
Chen M, Li Y, Birch D, Willows RD (2012) A cyanobacterium that contains chlorophyll f – a red-absorbing photopigment. FEBS Letters 586, 3249–3254.
| A cyanobacterium that contains chlorophyll f – a red-absorbing photopigment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVymsL3J&md5=fe41b60dac2a58e9c1ca3589a24bd310CAS | 22796191PubMed |
Chisholm SW, Frankel SL, Goericke R, Olson RJ, Palenik B, Waterbury JB, Lisa W-J, Zettler ER (1992) Prochlorococcus marinus nov. gen. nov. sp.: an oxyphototrophic marine prokaryote containing divinyl chlorophyll a and b. Archives of Microbiology 157, 297–300.
| Prochlorococcus marinus nov. gen. nov. sp.: an oxyphototrophic marine prokaryote containing divinyl chlorophyll a and b.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhsVyisLg%3D&md5=337198eb28826f00944d47914648162aCAS |
Corbet D, Schweikardt T, Paulsen H, Schmid VH (2007) Amino acids in the second transmembrane helix of the Lhca4 subunit are important for formation of stable heterodimeric light-harvesting complex LHCI-730. Journal of Molecular Biology 370, 170–182.
| Amino acids in the second transmembrane helix of the Lhca4 subunit are important for formation of stable heterodimeric light-harvesting complex LHCI-730.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmtVeit7g%3D&md5=610b97e125f174366795c6254fc61b01CAS | 17509613PubMed |
Croce R, van Amerongen H (2014) Natural strategies for photosynthetic light harvesting. Nature Chemical Biology 10, 492–501.
| Natural strategies for photosynthetic light harvesting.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpvFWks7Y%3D&md5=ce5a278b736e8de79b8d5997441a84f4CAS | 24937067PubMed |
Dau H, Haumann M (2008) The manganese complex of photosystem II in its reaction cycle – basic framework and possible realization at the atomic level. Coordination Chemistry Reviews 252, 273–295.
| The manganese complex of photosystem II in its reaction cycle – basic framework and possible realization at the atomic level.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXosFCitw%3D%3D&md5=f3566ca8e621a068bc0f602901b1c19aCAS |
Duxbury Z, Schliep M, Ritchie R, Larkum A, Chen M (2009) Chromatic photoacclimation extends utilisable photosynthetically active radiation in the chlorophyll d-containing cyanobacterium, Acaryochloris marina. Photosynthesis Research 101, 69–75.
| Chromatic photoacclimation extends utilisable photosynthetically active radiation in the chlorophyll d-containing cyanobacterium, Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXovFyhsr4%3D&md5=f6d8d1beb070a3ffb994de9bdc4349d5CAS | 19582591PubMed |
Evans HE, Foulds I, Carr NG (1976) Environmental conditions and morphological variation in the blue-green alga Chlorogloea fritschii. Journal of General Microbiology 92, 147–155.
| Environmental conditions and morphological variation in the blue-green alga Chlorogloea fritschii.Crossref | GoogleScholarGoogle Scholar |
Gan F, Zhang S, Rockwell NC, Martin SS, Lagarias JC, Bryant DA (2014) Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 345, 1312–1317.
| Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsV2qtLzF&md5=e042f763e636d0f3770d9504fb612e07CAS | 25214622PubMed |
Goericke R, Repeta DJ (1992) The pigments of Prochlorococcus marinus: the presence of divinyl chlorophyll a and b in a marine prochlorophyte. Limnology and Oceanography 37, 425–433.
| The pigments of Prochlorococcus marinus: the presence of divinyl chlorophyll a and b in a marine prochlorophyte.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XlsVKrsbs%3D&md5=b41964587d6a6c45e65643f750711b83CAS |
Gouterman M (1961) Spectra of porphyrins. Journal of Molecular Spectroscopy 6, 138–163.
| Spectra of porphyrins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3MXhtV2rtLw%3D&md5=54928e4bf8fa9dd1853704480060f41fCAS |
Hanson LS (1991) Molecular orbital theory of monomer pigments. In ‘Chlorophylls’. (Ed. H Scheer) pp. 993−1013. (CRC Press: Boca Raton, FL, USA)
Holt AS (1961) Further evidence of the relation between 2-desvinyl-2-formyl-chlorophyll-a and chlorophyll-d. Canadian Journal of Botany 39, 327–331.
| Further evidence of the relation between 2-desvinyl-2-formyl-chlorophyll-a and chlorophyll-d.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3MXntFegtQ%3D%3D&md5=c5c975201f9ee4fe83df62c778b050a4CAS |
Holt AS, Morley HV (1959) A proposed structure for chlorophyll d. Canadian Journal of Chemistry 37, 507–514.
| A proposed structure for chlorophyll d.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1MXovFCltQ%3D%3D&md5=3486af7d6a9e935e5bcc876235c03a85CAS |
Hoober JK, Eggink LL, Chen M (2007) Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynthesis Research 94, 387–400.
| Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtl2ksL7K&md5=edca1fe8fb4f7c413016a22c619346cfCAS | 17505910PubMed |
Hu Q, Miyashita H, Iwasaki I, Kurano N, Miyachi S, Iwaki M, Itoh S (1998) A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis. Proceedings of the National Academy of Sciences of the United States of America 95, 13319–13323.
| A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXntFWrsLc%3D&md5=39c8d2d5393fe6b6c47e8263cc4bcfa8CAS | 9789086PubMed |
Ito H, Tanaka A (2011) Evolution of a divinyl chlorophyll-based photosystem in Prochlorococcus. Proceedings of the National Academy of Sciences of the United States of America 108, 18014–18019.
| Evolution of a divinyl chlorophyll-based photosystem in Prochlorococcus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVOktLnE&md5=63b46c93bfb30dd097454a16f0040db7CAS | 22006316PubMed |
Itoh S, Mino H, Itoh K, Shigenaga T, Uzumaki T, Iwaki M (2007) Function of chlorophyll d in reaction centers of photosystems I and II of the oxygenic photosynthesis of Acaryochloris marina. Biochemistry 46, 12473–12481.
| Function of chlorophyll d in reaction centers of photosystems I and II of the oxygenic photosynthesis of Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFalurbK&md5=4dea4b28b362531ac773e3834e28e8e4CAS | 17918957PubMed |
Jeffrey ST, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie und Physiologie der Pflanzen 167, 191–194.
Kalyanasundaram K, Graetzel M (2010) Artificial photosynthesis: biomimetic approaches to solar energy conversion and storage. Current Opinion in Biotechnology 21, 298–310.
| Artificial photosynthesis: biomimetic approaches to solar energy conversion and storage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmslOht7w%3D&md5=4764cc1b7848a33d5dfeacb705199550CAS | 20439158PubMed |
Koehne B, Elli G, Jennings RC, Wilhelm C, Trissl H (1999) Spectroscopic and molecular characterization of a long wavelength absorbing antenna of Ostreobium sp. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1412, 94–107.
| Spectroscopic and molecular characterization of a long wavelength absorbing antenna of Ostreobium sp.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXks1CltbY%3D&md5=bc16bfd87cde817af8f9c2b9cd3b7766CAS |
Kühl M, Chen M, Ralph P, Schreiber U, Larkum AWD (2005) A niche for cyanobacteria containing chlorophyll d. Nature 433, 820
| A niche for cyanobacteria containing chlorophyll d.Crossref | GoogleScholarGoogle Scholar | 15729331PubMed |
La Roche J, Larkum AWD, Green BR, Van der Staay GWM, Partensky F, Ducret A, Aebersold R, Li R, Golden SS, Hiller RG, Wrench PM (1996) Independent evolution of the prochlorophyte and green plant chlorophyll a/b light-harvesting proteins Proceedings of the National Academy of Sciences of the United States of America 93, 15244–15248.
| Independent evolution of the prochlorophyte and green plant chlorophyll a/b light-harvesting proteinsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXntFKg&md5=e929ad9c2d151e7967f520bf2e56c45bCAS | 8986795PubMed |
Larkum AWD, Chen M, Li Y, Schliep M, Trampe E, West J, Salih A, Kühl M (2012) A novel epiphytic chlorophyll d-containing cyanobacterium isolated from a mangrove-associated red alga. Journal of Phycology 48, 1320–1327.
| A novel epiphytic chlorophyll d-containing cyanobacterium isolated from a mangrove-associated red alga.Crossref | GoogleScholarGoogle Scholar |
Li Y, Scales N, Blankenship RE, Willows RD, Chen M (2012) Extinction coefficient for red-shifted chlorophylls: chlorophyll d and chlorophyll f. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1817, 1292–1298.
| Extinction coefficient for red-shifted chlorophylls: chlorophyll d and chlorophyll f.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xkt1Ghs78%3D&md5=a5d5f5a8d5ad0e6a48808c35a72d37bbCAS |
Li Y, Cai Z-L, Chen M (2013) Spectroscopic properties of chlorophyll f. Journal of Physical Chemistry B 117, 11309–11317.
| Spectroscopic properties of chlorophyll f.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmsFGks7k%3D&md5=63a361b72317ae04fa41364f32c38528CAS |
Li Y, Lin Y, Loughlin PC, Chen M (2014) Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris–a filamentous cyanobacterium containing chlorophyll f. Frontiers in Plant Science 5, 67
| Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris–a filamentous cyanobacterium containing chlorophyll f.Crossref | GoogleScholarGoogle Scholar | 24616731PubMed |
Lin Y, Crossett B, Chen M (2013) Effects of anaerobic conditions on photosynthetic units of Acaryochloris marina. In ‘Photosynthesis research for food, fuel and the future’. (Eds T Kuang, C Lu, L Zhang) pp. 121−124. (Zhejiang University Press: Hangzhou, China)
Loughlin P, Lin Y, Chen M (2013) Chlorophyll d and Acaryochloris marina: current status Photosynthesis Research 116, 277–293.
| Chlorophyll d and Acaryochloris marina: current statusCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1ClsLzP&md5=e6fbc46c13a2e6494471f77631de976fCAS | 23615924PubMed |
Manning MM, Strain HH (1943) Chlorophyll d, a green pigment of red algae. Journal of Biological Chemistry 151, 1–19.
Marquardt J, Senger H, Miyashita H, Miyachi S, Mörschel E (1997) Isolation and characterization of biliprotein aggregates from Acaryochloris marina, a Prochloron-like prokaryote containing mainly chlorophyll d. FEBS Letters 410, 428–432.
| Isolation and characterization of biliprotein aggregates from Acaryochloris marina, a Prochloron-like prokaryote containing mainly chlorophyll d.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXktlCqtr4%3D&md5=5c41917e53b0c03c6bbee0cb4b846ab8CAS | 9237676PubMed |
Melkozernov AN, Blankenship RE (2003) Structural modeling of the Lhca4 subunit of LHCI-730 peripheral antenna in photosystem I based on similarity with LHCII. Journal of Biological Chemistry 278, 44542–44551.
| Structural modeling of the Lhca4 subunit of LHCI-730 peripheral antenna in photosystem I based on similarity with LHCII.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXoslWmu7Y%3D&md5=c91288061c872976e0d977ca1943461dCAS | 12923171PubMed |
Mielke SP, Kiang NY, Blankenship RE, Mauzerall D (2013) Photosystem trap energies and spectrally-dependent energy-storage efficiencies in the Chl d-utilizing cyanobacterium, Acaryochloris marina. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1827, 255–265.
| Photosystem trap energies and spectrally-dependent energy-storage efficiencies in the Chl d-utilizing cyanobacterium, Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitleit7c%3D&md5=d986801aa05fa65842ae8c5e591b4accCAS |
Miller SR, Augustine S, Olson TL, Blankenship RE, Selker J, Wood AM (2005) Discovery of a free-living chlorophyll d-producing cyanobacterium with a hybrid proteobacterial/cyanobacterial small-subunit rRNA gene. Proceedings of the National Academy of Sciences of the United States of America 102, 850–855.
| Discovery of a free-living chlorophyll d-producing cyanobacterium with a hybrid proteobacterial/cyanobacterial small-subunit rRNA gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXis1Cqtr8%3D&md5=2ea1c4eb5cd707f8bcdb8d041f6aaeedCAS | 15637160PubMed |
Mimuro M, Hirayama K, Uezono K, Miyashita H, Miyachi S (2000) Uphill energy transfer in a chlorophyll d-dominating oxygenic photosynthetic prokaryote, Acaryochloris marina. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1456, 27–34.
| Uphill energy transfer in a chlorophyll d-dominating oxygenic photosynthetic prokaryote, Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXotFKiuw%3D%3D&md5=e8aa8c53faf496cb3a6bdf945e7047e4CAS |
Mimuro M, Akimoto S, Gotoh T, Yokono M, Akiyama M, Tsuchiya T, Miyashita H, Kobayashi M, Yamazaki I (2004) Identification of the primary electron donor in PSII of the Chl d-dominated cyanobacterium Acaryochloris marina. FEBS Letters 556, 95–98.
| Identification of the primary electron donor in PSII of the Chl d-dominated cyanobacterium Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXoslyh&md5=31ee21aa5875ffa7d663bb583e8a770aCAS | 14706833PubMed |
Mitra AK (1950) Two new algae from Indian soils. Annals of Botany 14, 457–464.
Miyashita H, Ikemoto H, Kurano N, Adachi K, Chihara M, Miyachi S (1996) Chlorophyll d as a major pigment. Nature 383, 402
| Chlorophyll d as a major pigment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xmt1yktrk%3D&md5=9098a15a22d47fbb4c02414e10e59b98CAS |
Miyashita H, Ohkubo S, Komatsu H, Sorimachi Y, Fukayama D (2014) Discovery of chlorophyll d in Acaryochloris marina and chlorophyll f in a unicellular cyanobacterium, strain KC1, Isolated from Lake Biwa. Journal of Physical Chemistry & Biophysics 4, 149
| Discovery of chlorophyll d in Acaryochloris marina and chlorophyll f in a unicellular cyanobacterium, strain KC1, Isolated from Lake Biwa.Crossref | GoogleScholarGoogle Scholar |
Mohr R, Vosz B, Schliep M, Kurz T, Maldener I, Adams DG, Larkum ADW, Chen M, Hess WR (2010) A new chlorophyll d containing cyanobacterium: evidence for niche adaptation in the genus Acaryochloris. The ISME Journal 4, 1456–1469.
| A new chlorophyll d containing cyanobacterium: evidence for niche adaptation in the genus Acaryochloris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlWmtb3N&md5=9663762e9c7af85654e4108b06ba4d43CAS | 20505751PubMed |
Moore LR, Goericke R, Chisholm SW (1995) Comparative physiology of Synechococcus and Prochlorococcus: influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Marine Ecology Progress Series 116, 259–275.
| Comparative physiology of Synechococcus and Prochlorococcus: influence of light and temperature on growth, pigments, fluorescence and absorptive properties.Crossref | GoogleScholarGoogle Scholar |
Morel A, Ahn YH, Partensky F, Vaulot D, Claustre H (1993) Prochlorococcus and Synechococcus: A comparative study of their optical properties in relation to their size and pigmentation. Journal of Marine Research 51, 617–649.
| Prochlorococcus and Synechococcus: A comparative study of their optical properties in relation to their size and pigmentation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhsFyltb4%3D&md5=02c7677ce06bdd84ddcd254f4f2a30deCAS |
Murakami A, Miyashita H, Iseki M, Adachi K, Mimuro M (2004) Chlorophyll d in an epiphytic cyanobacterium of red algae. Science 303, 1633
| Chlorophyll d in an epiphytic cyanobacterium of red algae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXitFersb0%3D&md5=e6ab5b5578a78b9c74f2b7f89ee566e7CAS | 15016990PubMed |
Partensky F, Hoepffner N, Li WK, Ulloa O, Vaulot D (1993) Photoacclimation of Prochlorococcus sp. (Prochlorophyta) strains isolated from the North Atlantic and the Mediterranean Sea. Plant Physiology 101, 285–296.
Partensky F, Hess WR, Vaulot D (1999) Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiology and Molecular Biology Reviews 63, 106–127.
Pelletier PJ, Caventou JB (1818) Sur la matiere verte des feuilles. Annales de Chimie et de Physique 9, 194–196.
Petke JD, Maggiora G, Shipman L, Christoffersen R (1979) Stereoelectronic properties of photosynthetic and related systems-v. ab initio configuration interaction calculations on the ground and lower excited singlet and triplet states of ethyl chlorophyllide a and ethyl pheophorbide a. Photochemistry and Photobiology 30, 203–223.
| Stereoelectronic properties of photosynthetic and related systems-v. ab initio configuration interaction calculations on the ground and lower excited singlet and triplet states of ethyl chlorophyllide a and ethyl pheophorbide a.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXmslShtg%3D%3D&md5=7138274d12a12e496452284186ad58b1CAS |
Schlodder E, Çetin M, Byrdin M, Terekhova IV, Karapetyan NV (2005) P700+- and 3P700- induced quenching of the fluorescence at 760 nm in trimeric photosystem I complexes from the cyanobacterium Arthrospira platensis. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1706, 53–67.
| P700+- and 3P700- induced quenching of the fluorescence at 760 nm in trimeric photosystem I complexes from the cyanobacterium Arthrospira platensis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFGht77L&md5=0a87b737a71acb9f81b8c62a09522714CAS |
Tomo T, Okubo T, Akimoto S, Yokono M, Miyashita H, Tsuchiya T, Noguchi T, Mimuro M (2007) Identification of the special pair of photosystem II in a chlorophyll d dominated cyanobacterium. Proceedings of the National Academy of Sciences of the United States of America 104, 7283–7288.
| Identification of the special pair of photosystem II in a chlorophyll d dominated cyanobacterium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXls1OgtLs%3D&md5=5da6c55b40ce02dd32bf286dd670ac57CAS | 17431035PubMed |
Tomo T, Kato Y, Suzuki T, Akimoto S, Okubo T, Noguchi T, Hasegawa K, Tsuchiya T, Tanaka K, Fukuya M, Dohmae N, Watanabe T, Mimuro M (2008) Characterization of highly purified photosystem I complexes from the chlorophyll d-dominated cyanobacterium Acaryochloris marina MBIC 11017. Journal of Biological Chemistry 283, 18198–18209.
| Characterization of highly purified photosystem I complexes from the chlorophyll d-dominated cyanobacterium Acaryochloris marina MBIC 11017.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsVCnsrk%3D&md5=2a5d8fd91eba765bdb22e7902c21ef0aCAS | 18458090PubMed |
Tomo T, Allakhverdiev SI, Mimuro M (2011) Constitution and energetics of photosystem I and photosystem II in the chlorophyll d-dominated cyanobacterium Acaryochloris marina. Journal of Photochemistry and Photobiology. B, Biology 104, 333–340.
| Constitution and energetics of photosystem I and photosystem II in the chlorophyll d-dominated cyanobacterium Acaryochloris marina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntFWjsbo%3D&md5=e4b4563b5d8191301315e99177d0dcacCAS | 21530298PubMed |
Weiss C (1978) Electronic absorption spectra of chlorophylls. In ‘The porphyrins. Vol. III, Physical chemistry. Part A’. (Ed. D Dolphin) pp. 211–223. (Academic Press: New York)
Wilhelm C, Jakob T (2006) Uphill energy transfer from long wavelength absorbing chlorophylls to PSII in Ostreobium sp. is functional in carbon assimilation. Photosynthesis Research 87, 323–329.
| Uphill energy transfer from long wavelength absorbing chlorophylls to PSII in Ostreobium sp. is functional in carbon assimilation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksFGlur4%3D&md5=aad8d1a55a3082c5d7eb7526ac897f0eCAS | 16416051PubMed |
Willows RD, Li Y, Scheer H, Chen M (2013) Structure of chlorophyll f. Organic Letters 15, 1588–1590.
| Structure of chlorophyll f.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktFKgt74%3D&md5=537005d9867bd4d140d578d54bbb8869CAS | 23496297PubMed |
Zapata M, Garrido JL, Jeffrey SW (2006) Chlorophyll c pigments: current status. In ‘Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications’. (Ed. B Grimm) pp. 39−53. (Springer: Dordrecht, The Netherlands)



