Rapid stomatal response to fluctuating light: an under-explored mechanism to improve drought tolerance in rice
Mingnan Qu A , Saber Hamdani A , Wenzhen Li C , Shimei Wang B , Jiuyou Tang C , Zhuo Chen C , Qingfeng Song A , Ming Li A , Honglong Zhao A , Tiangen Chang A , Chengcai Chu C and Xinguang Zhu A DA CAS-Key Laboratory for Computational Biology, CAS-MPG Partner Institute for Computational Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Room 106, Physiology Building, 320 Yueyang Road, Shanghai 200031, China.
B Anhui Agricultural Academy of Sciences, 40 Nongke South Road, Hefei, Anhui 230031, China.
C The State Key Laboratory of Plant Genomics, Institute of Genetics and Developmental Biology, 1 Beichen South road, Chinese Academy of Sciences, Beijing 100101, China.
D Corresponding author. Email: zhuxinguang@picb.ac.cn
Functional Plant Biology 43(8) 727-738 https://doi.org/10.1071/FP15348
Submitted: 8 November 2015 Accepted: 29 February 2016 Published: 23 June 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Light inside a canopy constantly fluctuates. Under fluctuating light (FL) conditions, stomatal conductance and photosynthetic rate constantly change. In this study, we explored whether this dynamics of stomata movements upon FL influenced the water use efficiency of rice in the field. We used a USDA-curated rice mini-core diversity panel consisting of 204 worldwide distributed accessions. A priori model on dynamic stomatal response to FL was utilised to identify kinetic parameters describing the stomatal delays during the closing (τcl) and the opening (τop) phase. Result showed that τcl had a larger variation than τop across the mini-core panel. τcl was negatively correlated with water use efficiency (WUE) related traits, stem diameter, grain weight per tiller and heading time, but positively correlated with maximum annual temperature, carbon assimilation related traits and biomass (P < 0.05). We further showed a strong correlation of τcl with the relative decrease of biomass under drought in 14 accessions with different τcl. We discussed the adjustment of stomatal conductance under fluctuating light in light of the trade-off between optimising CO2 uptake and optimising water saving. This study suggests that stomatal dynamics under fluctuating light is closely related to drought resistance and hence detailed study is needed to enable its application in breeding drought tolerance in rice.
Additional keywords: abiotic stress, acclimation, drought stress, photosynthetic rate, stomatal conductance, yield.
Introduction
Light levels in a canopy fluctuate frequently and rarely stay at constant levels. The passing cloud can create intermittent shade to leaves in a canopy (Knapp and Smith 1988). Leaves at the top of a canopy can shade leaves at lower layers of a canopy. When the wind blows, leaves at lower layers of a canopy experience more fluctuating light (FL) levels or sunflecks (Amtmann 1985; Way and Pearcy 2012). Even if leaves in a canopy keep still, the gradual changes of the sun-earth geometry during a day can create FL on leaves at lower layers of a canopy (Myneni and Impens 1985). Although FL may only last from seconds to minutes, FL can contribute up to 20–80% of the total solar energy incident on understory leaves (Chazdon 1988; Pearcy 1990).
Possibly as a result of long-term plant evolution under dynamic light conditions, plants are equipped with a diverse set of mechanisms to gain increased fitness or to avoid damage under FL levels (Stitt and Zhu 2014). For example, under strong light, state transition can occur to transfer energy away from PSII to avoid photo-damage (Dietzel et al. 2011). Chloroplast movement is another commonly observed mechanism plants utilise to avoid photo-damage (Kasahara et al. 2002). In addition to these relatively slow structural or anatomical changes, plants also use an array of biochemical and biophysical mechanisms to cope with FL. Under strong light, the rapid decrease of lumen pH can lead to increased rate constant for heat dissipation, which can help quickly dissipate light energy that are in excess of the capacity of photochemistry (Ort 2001). When FL occur, the rate constant of heat dissipation decreases and helps gain increased canopy light use efficiency (Zhu et al. 2004). The understory plants are capable of accumulating large amount of 3-phosphate glycerate, which can be used to utilise FL (Pearcy 1990).
Recently, another mechanism that may be important for controlling plant light and WUE in the field has been recognised: the rapid rate of responses of stomata conductance to FL levels (Lawson and Blatt 2014). The theory is that when the light level decreases, there will be a potential decrease of WUE if stomatal conductance (gs) cannot decrease rapidly; however, when the light levels increase abruptly from low to high light levels, if gs cannot increase rapidly, photosynthetic light use efficiency will be decreased. There are large variations in both the sensitivity and responsiveness of gs to changing environments among different species (Lawson 2009; Lawson et al. 2003, 2012).
Applying a proper mathematical modelling to qualify dynamic response in gs to FL is critical to identifying variations among or inter-species. There are several strategies to estimate dynamic changes in gs to FL treatments, such as piece-wise linear, logistic models, asymmetric sigmoid function and exponential models (Kirschbaum et al. 1988; Zipperlen and Press 1997; Naumburg et al. 2001; Vico et al. 2011; Vialet-Chabrand et al. 2013). Notably, Vico et al. (2011) first introduced two parameters – τcl and τop – to represent stomatal response delays during closing and opening phase respectively. Vico et al. (2011) obtained these parameters by exponential fitting on 40 dataset from the public resource. In most species, delays in stomatal opening appear to be shorter than delays in closing, that is τop < τcl (Ooba and Takahashi 2003; Vico et al. 2011). Time scales of stomatal delays in different species also exhibit a large degree of variation. This variation was reported to be commensurate with FL durations (Cardon et al. 1994; Naumburg et al. 2001) and to depend on the time of day and history of FL occurrence (Kaiser and Kappen 1997).
Laboratory studies have shown that leaves with more rapid responses of gs exerted higher leaf-level water and light use efficiencies (Lawson and Blatt 2014). Considering the high level of temporal and spatial heterogeneities of light inside a canopy, it is predicted that plants with more rapid response to changes in light levels should have increased water and light use efficiencies in the field. In addition, plant functional types and geographic origins are two important determinants of gs delays in response to FL (Vico et al. 2011). Therefore, in this work, this hypothesis has been tested with a rice diversity panel that was developed by USDA to represent the genetic diversity of the 18 000 accessions of the USDA-ARS rice (Oryza sativa L.) germplasm collection (Agrama et al. 2009). This collection contains 217 accessions which were originated from 76 countries covering 15 geographic regions. It consists of six groups, indica (35.4%), aus (18.7%), tropical japonica (18.2%), temperate japonica (15.2%), aromatic (3.0%) and their admixtures (9.6%) (Agrama et al. 2009; Li et al. 2010). This mini-core collection, with its large genetic diversity and large diversity of climates of their origins, represents an ideal choice of germplasm to study the natural variation of stomatal delays and its implication to both water and light use efficiencies in the field.
In this study, we aim to address three specific questions. (1) What are the levels of variability in stomatal responses to FL in the rice mini-core diversity panel? (2) Do plants with different stomatal responses to FL have different WUE? (3) Do plants with different stomatal responses to FL have different drought tolerance in the field?
Materials and methods
Plant materials
The experiments were conducted in 2013 and 2014. In July 2013, a diversity panel composed of 204 accessions derived from 217 USDA mini-core rice accessions. The remaining 13 accessions in the mini-core accessions were not used due to long growth seasons.
Geographic and climate information of the origins of different accessions
Data of geography such as latitude (LAT), longitude (LONG) and elevation (ELV) for the origin of each accession was collected from Genetic Stock Oryza (http://www.ars.usda.gov/Main/docs.htm? docid˛ = Í8318, accessed 3 May 2014), according to Genetic stock number (GSOR) of each accession. Annual climate information such as mean (MET) and maximum (MAT) annual temperature (°F), mean annual dew point (MEDP) and mean annual precipitation (PRCP) was downloaded from National Oceanic and Atmospheric Administration, NOAA (https://gis.ncdc.noaa.gov/, accessed 15 March 2015). All of these data were collected based on the year that each accession released (1914–97) to determine originated weather condition for each accession. Total photosynthetically active radiation (PAR), and maximum daylength (MDL) during growth season were determined by the solar elevation angle, which further depends on the originated information of LAT and LONG for each accession (Song et al. 2013).
Growth conditions
The experiments using the diversity panel with 204 accessions were conducted in the Institute Genetics and Developmental Biology, Chinese Academy Sciences (CAS), Beijing (116.3943°E, 39.9820°N) in 2013. Rice seeds from the each accession were grown for ~30 days in a soil seed bed. Then, from early June, seedlings were transplanted into plastic pots (12 L volume) containing commercial peat soil (Pindstrup Substrate no. 4, Pindstrup Horticulture Ltd, Shanghai, China) and then grown under outdoor conditions. Three plants for each accession were seeded in one pot and each accession used two pots. Measurements were started from early July 2013. During this period, the average lower temperature range was 24~26°C, and the average higher temperature range was 30~31°C, whereas the humidity range was 61~75%. Before the measurements, plants were first moved into an environment-controlled growth room for 30–50 min to ensure full-adaptation of stomata. The growth room was maintained to have an air temperature of 27°C and a photon flux density of around 600 μmol m–2 s–1. The experiments were conducted between 0830 to 1630 hours.
Based on the measured stomatal delays in response to fluctuating light (FL) levels (see gas exchange measurements for more details) from 2013, a subset of 204 rice accessions, i.e. 12 accessions with extremely contrasting τcl was selected to determine whether different τcl is able to affect a drought treatment study in 2014. This selection strategy is based on larger amplitude and longer duration in τcl than that τop (Ooba and Takahashi 200; Vico et al. 2011). Therefore, in 2014 experiments, our study focussed on τcl instead of τop. Among these 12 accessions, six were in fast τcl group, six were in slow τcl group (Table 1). Between two τcl groups, the MAT were significantly different (P < 0.05). MAT in fast τcl group exhibited a lower value compared with slow τcl group.

|
In 2014, these 12 rice accessions with contrasting τcl based on previous investigation in 2013 were grown under two conditions, i.e. an extreme drought stress condition (DS hereafter) and a normal irrigated condition (NORM hereafter) in Hainan (110.0375°E, 18.5060°N), China. For the drought treatment, rice was sheltered during rain. The mean daily air temperature between two locations during growth seasons (Beijing and Hainan) were 25.8 and 26.5°C. Mean daylengths were 12.5 and 12 h respectively. These data was obtained from China Meteorological Administration (http://www.cma.gov.cn/, accessed 15 March 2015) recorded from 2013 to 2014. Two plots were used for the DS conditions and one plot was used for the NORM condition. To decrease the boundary effect, 36 plants were planted (6 × 6) for each accession, with 15 cm between plants within each row and 20 cm between rows. The rice fields were managed according to standard local agronomic practice with the following fertiliser application guideline: 48 kg N ha–1, 120 kg P2O5 ha–1, and 100 kg K2O ha–1 as the basal fertiliser, and additional 86 kg N ha–1 at the tillering stage and 28 kg N ha–1 at the booting stage. Weeds in both DS and NORM fields were controlled by a combination of chemical and manual methods and insects were controlled chemically. For DS blocks, soil moisture was monitored with a time domain reflectometry method at a soil depth of 20–30 cm (Ledieu et al. 1986). For the DS blocks, the soil water content was 8~12% (v/v) during photosynthetic measurements.
Fluctuating light treatments
Experiments were conducted between 50 and 60 days after emergency (DAE) in both 2013 and 2014. The middle segment of the top fully expanded leaf on the main tiller was chosen during experiments. Four portable infrared gas exchange system (Li-6400XT, Li-Cor Inc., Lincoln, NE, USA) were pre-adjusted by manufacturer standard gas and used simultaneously during experiments. The chamber is 2 × 3 cm in dimension and is equipped with a blue–red LED light source. The measurements were conducted on sunny days between 0900 and 1130 hours and between 1300 and 1500 hours. During the measurements, the leaf temperature was maintained at 25°C and the RH was maintained in range of 68 to 87%. The reference CO2 concentration was set as 400 μmol mol–1 during the experiment.
During the fluctuating light (FL) treatments, leaf was first exposed to a high light with a PPFD of 1200 μmol m–2 s–1 for at least 5 min until it reaches a steady-state. Then the PPFD was switched to be 100 μmol m–2 s–1. In 2013, the plants were kept under this low light for 25 min while in 2014 plants were kept under low light for 20 min. The light level of 100 μmol m–2s–1 was chosen based on work by Lawson and Blatt (2014). Leaves were kept under low light until gs stabilised. After the gs stabilisation, the PPFD was recovered to 1200 μmol m–2 s–1. Leaves were kept under high light for 5 min. Four biological replicates from four different plants for each accession were determined. With the measured A and gs, we calculated the ratio of A (and gs) between DS and NORM conditions, i.e. ADS/ANORM and gsDS/gsNORM, where ADS and ANORM represents the A under DS and NORM conditions, and gsDS and gsNORM represents the gs under DS and NORM conditions.
Calculation of photosynthetic and physiological parameters
In this study, 13 photosynthetic traits during FL were determined including steady-states and instantaneous photosynthetic rates. The initial WUE, gs and A under high light as well as these three photosynthetic parameters under low light were estimated as the average values of these parameter during the last five measurements before light switching. We estimated the ratio in A under high light over low light (Aloss) as function of light use efficiency of A. Same equation was applied in WUE (WUEloss) as well. As gs exhibits an asymmetric response to light changes, we used an exponential fitting via MATLAB R2010a (MathWorks Inc., Natick, MA, USA), to determine amplitude of stomatal delays when light switching according to Vico et al. (2011) using the following equation:
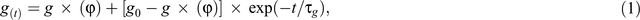
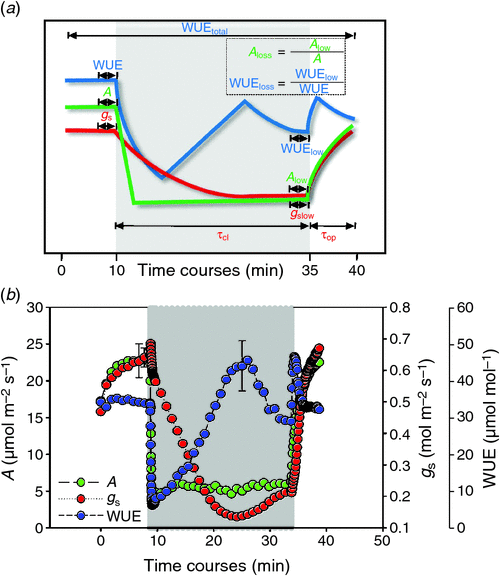
where τg takes different values depending on whether the leaf is responding to a sudden increase (τop) or decrease (τcl) in light. The regions used to calculate τcl and τop are shown in Fig. 1a. These two parameters represent stomatal delays in minute scale. Goodness of fit (R2) after simulation in stomatal opening phase and stomatal closing phase were 0.88 and 0.95, respectively, which reveals a good fitting algorithm applied in this study. To decrease initial effects of gs on τcl, τcl over gs was calculated. To compare the potential decrease of WUE due to difference in the gs responses, we estimated WUEtotal by obtaining the averaged WUE throughout the light fluctuating experiment. We determined the SPAD value using the SPAD 502 Plus Chlorophyll meter (Spectrum Technologies Inc., Aurora, IL, USA). For each leaf, averaged SPAD values were recorded from five different positions in the middle section, and four replicates from four different plants were determined for each accession.
|
Agronomic traits measurements
In 2013 large-scale measurements, four spike related traits, i.e. final tiller number (TIR), stem base diameter (SBD), spike length (SPL) and number per plant (SPN), and three grain weight related traits, i.e. yield per plant (YD), yield per tiller (YD/TIR) and heading time (HEAD) were determined. At least four replicates were conducted for each trait above.
The total aboveground biomass for each accession was measured with a sample size of four while the gas-exchange data were collected. After determining FW, samples were kept at 120°C for 1 h and then under 70°C for at least 24 h until constant weight in baking oven. In 2014 experiments, DW under NORM over drought conditions were calculated to determine biomass accumulation in response to DS at canopy levels.
Isotope carbon ratio measurements
To evaluate the long-term WUE, we measured the carbon isotope ratio (δ13C) of the top fully-expanded leaf at the maturity stage (around 90 DAE). To do this, leaf materials were first dried at 70°C to constant weight, then grounded and analysed using Thermo Delta V advantage. The long-term standard deviation of the equipment is 0.1‰. The 13C/12C ratio was expressed following the delta (δ) notation in parts per mill (‰) relative to the international V-PDB (Vienna PeeDee Belemnite) standard. The measurements were performed at the Mass Spectrometry Laboratory of Forestry Science Research Institute China. Four replicates were used for δ13C measurements.
Statistical analysis
To analyse the variance of 24 physiological traits and 9 originated climate information in 204 mini-core collection, one group variance analysis was conducted using StatView, ver. 5.0.1 (SAS Institute Inc., Cary, NC, USA). Coefficient of variance and standard deviation were calculated when comparing groups with or without same unit. The correlation between traits was calculated as Pearson’s correlation coefficients among genotype means. Corrplot package in R software (http://www.r-project.org/, accessed 13 June 2015) was employed to calculate Pearson’ coefficient index (r) or goodness of fit (R2).
Results
Natural variations of the rapidity of responses of gs to fluctuating light levels
During fluctuating light (FL), A, gs and WUE showed dynamic changes (Fig. 1b). Steady-state A were 23.02 ± 2.96 μmol m–2 s–1 at 1200 μmol m–2 s–1 (Fig. 1b; Table 2). After decreasing PPFD from 1200 to 100 μmol m–2 s–1, A decreased immediately by 80% within 1 min and kept 4.87 ± 0.64 μmol m–2 s–1, whereas gs showed an exponential reduction: gslow across mini-core accession was 0.12 ± 0.04 μmol m–2 s–1, which accounts for 25% of the initial gs (Fig. 1b). However, WUE increased in an opposite pattern as the change in gs, and reached a maximum at around 13 min after switching to low light. When the light level was increased again, a rapid increase in A, gs and WUE was observed.

|
In the 2013 experiments, we investigated nine eco-physiological traits and eight biomass related traits. To further analyse whether stomatal responses might be caused by different eco-niches in which plants were originally grown, we obtained the 13 geography and climate factors related to the growing regions of these rice accessions from NOAA database (https://gis.ncdc.noaa.gov, accessed 15 March 2015). The fold differences between the maximum and minimum for each geographical factor were more than those of the ecophysiological and biomass traits. Most of these traits showed a significant difference (P < 0.0001), except for maximum daylength (MDL) according to one-way ANOVA (Table 2). Across these geographical factors, annual precipitation (PRCP) and maximum air temperature (MAT) exhibited 676- and 14.1-fold differences between the maximum and minimum respectively. Among them, latitude (LAT) and longitude (LONG) were in the range from –34.9 ~50.8°, and –99.2 ~178.7° respectively. Annual mean temperature (MET) ranged from 31.2 ~91.8°F. MAT ranged from 9 ~76.5°F.
There were large natural variations in τcl and τop (Fig. 2). The values in τcl ranged from 1.81 to 12.42 with a normal distribution across these 204 rice accessions (Fig. 2; Table 2). Among subpopulation, AUS exhibited a relatively higher median and minimum values of τcl and τop, compared with two types of japonica (TEJ and TRJ) (Fig. 2). The median of τcl and τop across indica (IND) showed similar or higher rates than in japonica. Mean of τcl was 4.46, which is two times higher than τop. Fold differences between minimum and maximum in τcl and τop were 6.86 and 3.24, respectively, the variance level is significant (P < 0.0001) (Table 2). To eliminate the effects of initial gs, τcl was normalised (τcl/gs) in this study. The τcl/gs ranged from 0.28 to 2.36 with an 8.42-fold difference (Table 2). With respect to fold difference in A, gs and WUE under both high light and low light, the ranking from high to low is: gs > WUE > A, i.e. a relatively higher fold difference was observed in gs under high light and low light, which were 5.5 and 5.2 respectively. Whereas A under high light and low light exhibited a relatively low differences, the fold difference is no more than 2.5. Among ecophysiological traits, SPAD values exhibited highest standard deviation and coefficient of variance.
Under different geography and climate conditions, biomass related traits exhibited a significant variation as well (P < 0.0001). FW and DW per plant ranged from 48.6 to 350.3 and 9.8 to 80.1 g respectively (Table 2). In terms of spike traits, i.e. SBD, TIR and SPL, the fold differences between the minimum and maximum were 3.96, 5.49 and 1.82 respectively. In contrast, YD and YD/TIR exhibited higher fold differences, i.e. 8.68 and 5.60 respectively. HEAD time ranged from 64 to 150 days. The mean of HEAD time was 113 days.
Variation of τcl is linked to water and shadefleck use efficiency in the mini-core panel
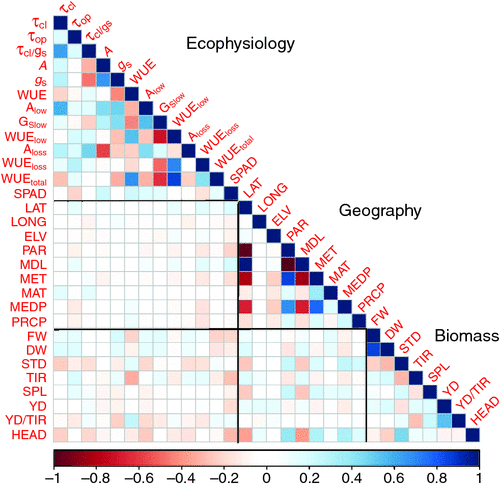
Among the collected parameters, we calculated the Pearson correlation coefficient (r) between them (Fig. 3; see Table S1, available as Supplementary Material to this paper). Correlation r in photosynthetic traits during FL revealed a robust relationship between these traits. τcl was positively correlated with τop, τcl/gs, A, gs, Alow, gslow and Aloss (P < 0.05), but was negatively correlated with WUE and WUEtotal (P < 0.05). Furthermore, τop was positively correlated with WUEloss, WUElow and WUEtotal (P < 0.05), but not significantly correlated with A, gs and WUE. And τop was negatively correlated with SPAD (P < 0.05).
|
The diverse origin, from the perspective of geography and year of release, in the mini-core collection can affect stomatal evolution under FL. As shown in Fig. 3, τcl and τop displayed an intricate relationship in response to geography factors, τcl and τcl/gs was positively correlated with MAT (P < 0.05), whereas τop was positively correlated with LAT and MAL (P < 0.05) but negatively correlated with MEDP (P < 0.05) that is typically representing absolute air humidity.
For the biomass related traits, both τcl and τop were positively correlated with biomass accumulation (DW and FW) (P < 0.05), but no correlation was observed between τcl/gs and biomass (Fig. 3). In contrast, τcl, τop and τcl/gs were all negatively correlated with SBD. In terms of spike traits, τop was positively correlated with TIR and SPL, but negatively correlated with YD/TIR (P < 0.05). In contrast, τcl was negatively correlated with HEAD time (P < 0.05).
The relationship between τcl and drought resistance in the field
Because τcl was negatively correlated with WUE related traits (Fig. 3) and showed a larger variation compared with τop (Fig. 2), we selected 12 accessions for further experiment in 2014 to test whether difference in τcl can lead to difference in WUE in the field (see material and methods for details). Among these 12 accessions, six accessions possessed extremely high six τcl (Fast group) and six accessions with extremely low six τcl (slow group).
In the 2013 experiments, A and gs within the slow group were 30% and 80% higher than those in the fast group respectively before light was switched from high to low light (Fig. 4a, b). After light was switched to low light, gs in the fast group declined and gained stable values within 8 min. In contrast, there was a greater lag in gs in the slow group. A under low light didn’t differ between two groups. When light was increased from low to high light levels, gs and A in two groups increased rapidly. Before light switching, WUE in the fast group was ~75 μmol mmol–1, which is 50% higher than that in slow group. After light switching to low light levels, WUE dropped sharply then recovered back after a new balance between A and gs was formed. WUElow in both groups were slightly lower compared with WUE under high light. However, WUE in fast group recovered to normal levels at a faster pace than that in the slow group. In terms of Ci/Ca (Fig. 4d), because of stomatal effects, a relatively higher Ci/Ca was induced in the slow group than the fast group after light switching. The dynamics in A under FL was similar between the fast and slow groups; however, the dynamics of gs differ dramatically between the fast and slow group (Fig. 4, see Fig. S1, available as Supplementary Material to this paper).
The τcl measured across 12 accessions under two growth locations (Beijing, BJ and Hainan, HN) were highly correlated (Fig. S3). R2 regarding to τcl between BJ and HN was 0.65; similarly, R2 for τcl/gs between BJ and HN was 0.79 (P < 0.001) (Fig. S6). In addition, τcl hold the higher values in slow group than that that of the fast group under two growth conditions (Fig. S3). A co-ordination in τcl and a conserved ranking across accessions between NORM and DS condition were observed (Fig. 5, Fig. S3). In contrast, the τop showed a slightly less conserved ranking between these two conditions (Fig. 5b). Furthermore, when the τcl/τop in the DS was plotted against the ratio in NORM (Fig. 5c), a slope <1 was obtained, i.e. we have a smaller τcl/τop under DS compared with the ratio under NORM.
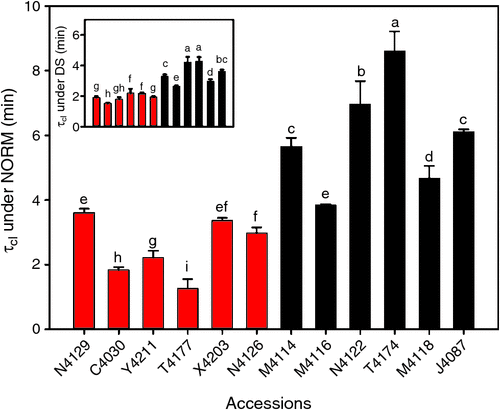
Among accessions, τcl appeared to be substantial differences. In particular, T4174 and C4030 showed an extremely contrasting τcl with around 3-fold differences under both NORM and DS conditions (Fig. 6). Diverse response across accessions due to DS was observed. Drought treatments induced 2-fold differences in τcl in T4174, but 50% fold differences in T4177 (Fig. 6). A positive correlation between τcl and biomass (DW) under both NORM and DS conditions was observed (R2 = 0.67 and 0.37 respectively) (Fig. 7c and insert in Fig. 7c). Remarkably, both τcl was negatively correlated with biomass drought ratio (Fig. 7d). The R2 is 0.69, while R2 of τcl/gs is 0.43 (Fig. S6). In contrast, A and gs across 12 subset accessions were not significantly correlated with biomass drought ratio (Fig. S4).We further evaluated the correlation between biomass drought ratio with the changes of A and gs under drought stress. Specifically, we calculated the ratio of A (and gs) between DS and NORM conditions, i.e. ADS/ANORM and gsDS/gsNORM, and the obtained the correlation between biomass drought ratio and ADS/ANORM and gsDS/gsNORM separately. The biomass drought ratio showed a higher R2 with τcl than either A, or gs or ADS/ANORM or gsDS/gsNORM (Figs 7d, S5). These results suggested that τcl, instead of either A or gs or ADS/ANORM or gsDS/gsNORM, was closely related to drought tolerance. Biomass between 2013 and 2014 experiments showed a robust correlation (R2 = 0.88) (Fig. S2).
|
Drought induced ~60% reduction in biomass on average across accessions (data not shown), but changes in biomass under DS differed among accessions obviously from each other (Fig. 7b). Among these accessions, biomass in T4174 showed a relatively higher values; in contrast, C4030 showed a lower biomass (Fig. 7a). In terms of biomass drought ratio, N4129 and Y4211 were around 0.75, which are 2-fold higher than T4174 and N4122 (Fig. 7b).
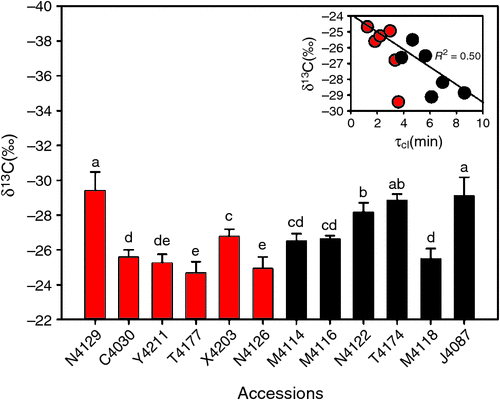
Correlation between δ13C and τcl
To investigate whether τcl can affect long-term leaf water use efficiency, carbon isotope ratio, δ13C in 12 accessions were determined. Result showed a negative correlation between τcl and δ13C (R2 = 0.5) (Fig. 8 insert). δ13C across accessions were in ranges of –24.9 to –29.4‰. Accessions in fast group exhibited a lower values in δ13C, compared with that in slow group (P = 0.05). In particular, δ13C of T4174 and J4087 in slow group were averaged around –29.0‰, in contrast, the average δ13C of Y4211 and N4216 in fast group were –25.1‰ (Fig. 8). The τcl showed a positive correlation with biomass and a negative correlation with the biomass drought ratio, i.e. the ratio of the biomass under drought to the biomass under control conditions (Figs 6, 7c, d).
|
Discussion
Stomata always exhibited a lag before getting stabilised during fluctuating light (FL) regimes, whereas carbon assimilation (A) responded to FL at a faster pace (Pfitsch and Pearcy 1989; Vico et al. 2011). This study used a rice mini-core panel consisting of 204 accessions collected by USDA to examine the significance of this phenomena to drought resistance in the field. The results suggest that there were large natural variations in the stomatal delays in response to FL and this variation was closely related to WUE and drought resistance in the field.
There were substantial natural variations in the stomatal delays to changes in light levels in the rice diversity panel (Fig. 2; Table 2), which is consistent with earlier reports (Chazdon 1988; Pearcy 1990; Ooba and Takahashi 2003). Simulation studies (Vico et al. 2011) using different species assembled from public database showed τop is less than that τcl during FL in graminoids, forbs and angiosperms, and there is a strong coordination between τcl and τop. Rice in our study showed the same trend as well (Fig. 3; Table 2). The value of τcl across these 204 rice accessions varied between 1.81 and 12.42 (Table 2) whereas the values of τop ranged between 1.21 and 3.93 (Table 2). This much higher value of τcl compared with τop (Table 2; Fig. 2) suggested that rice has the tendency of optimising carbon assimilation instead of conserving water under NORM conditions. This might be related to the fact that rice normally is grown in wet environments, therefore, the evolutionary pressure is on selection of faster stomatal opening to optimise the photosynthetic light use efficiency. Consistent with this, a survey of light saturated rate of photosynthesis in 24 accessions of rice (Amax), representing 17 species and 8 genomes, showed that Amax ranged from 25 to 45 μmol m–2 s–1 (Giuliani et al. 2013), which is relatively higher compared with Amax of other C3 plants (Wright et al. 2004).
Compared with the NORM conditions, rice grown under DS conditions showed decreased τop and τcl (Fig. 5a, b). But the decrease in τcl was higher than τop, as shown by the decreased ratio of τcl/τop under DS conditions (Fig. 5c). This indicated that the internal regulatory mechanisms of the control of stomatal conductance conditions to optimise water conservation instead of carbon assimilation under DS. For rice grown in a paddy field, the relatively faster opening of stomata compared with the closing of stomata (i.e. high τcl/τop) under FL can lead to a relatively less favourable WUE at the top of the canopy where the leaves experience relatively constant high light levels.
The observed variations of both parameters should be related to the long-term adaptation to the climatic conditions of the eco-niches, as demonstrated by the correlation between these parameters and the geographic and climate factors of the origin of rice accessions (Fig. 3). For example, τcl was positively correlated with MAT (P < 0.05), but τop was negatively correlated with MEDP (P < 0.05) and PRCP. The positive correlation between τcl and MAT was in contrast to the expectation that under high temperature regions a lower τcl would be desirable to ensure higher WUE. A higher dew point indicates higher humidity in the air. In our study, the negative correlation between τop and MEDP suggested that areas with high humidity, i.e. high MEDP, will have a lower τop, i.e. faster stomatal opening speed. In line with this, PRCP showed a negative correlation with τop, i.e. under high precipitation, the τop would be lower. These are reasonable since under conditions without water stress, decrease τop, i.e. increasing the stomatal opening speed can help optimise photosynthetic light use efficiency, which is more important than decreasing the stomatal response time for water saving. Therefore, humidity and precipitation are dominant environmental parameters that influence the speed of stomatal dynamics under varying light conditions.
Large variations in τcl and τop between different species have been observed previously, this study showed that not only there is also a huge variation of τcl and τop within the same species, but also there are substantial variation between subgroups of rice. For example, the AUS showed relatively higher τcl and τop while TRJ showed low values of τcl and τop (Fig. 2). These rice accessions therefore provide a rich source of materials to study the potential driving force and molecular mechanisms controlling the evolution of these different features.
The large variation in τcl is closely related to drought resistance in the field. The relationship between τcl and WUE has been reported earlier at the leaf level (Lawson and Blatt 2014). However, the potential impacts of different stomatal dynamics on drought resistance in the field will depend the time scale of the dynamic changes of the light environments inside a canopy, which will be further determined by the canopy architecture, wind speed, cloud movement, angle of incident radiation etc. (Chazdon 1988; Pearcy 1990). Data from this study demonstrate that accessions with higher τcl, i.e. slower rate of stomatal closure, exerted a lower drought resistance, as demonstrated by the relatively higher biomass drought ratio (Fig. 7b, d). This is further confirmed by the changes of isotope carbon ratio, δ13C (Fig. 8), which reflects the long-term WUE of a plant (Farquhar and Richards 1984; Farquhar et al. 1989). We further tested whether the drought resistance might also be linked to the difference in A, gs, or changes of A or gs under drought conditions (i.e. ADS/ANORM, or gsDS/gsNORM). Correlation analysis suggested that in the biomass drought ratio possessed much higher correlation with τcl as compared with those with either A, or gs or ADS/ANORM, or gsDS/gsNORM (Figs 7, S4, S5). The biomass ratio showed a R2 of 0.2 with gsDS/gsNORM. Therefore, though the data also indicated that gs might also be related to the biomass drought ratio, τcl is a parameter that are more closely related to drought resistance in the field.
Our findings suggest that τcl exhibited a positive correlation with biomass accumulation, though decreased τcl was linked to an increased drought resistance. Indeed, both τcl and τop were positively correlated with biomass accumulation (DW and FW) (Fig. 3). Concurrent with this positive correlation, we also found a positive correlation between τcl and gs and A (Fig. 3), i.e. those lines with a faster stomatal closing dynamics indeed performed on average lower gs and A values. The observed correlation between τcl and biomass therefore may be related to the overall higher A in those lines with higher τcl. The molecular mechanism underlying the positive correlation between τcl and A and gs is unknown. However, these data suggest that for regions where drought is relatively a rare event, decreasing τcl should not be used as a breeding target. In contrast, for regions with frequent severe drought, it would be more effective for rice productivity (Tuberosa and Salvi 2006). This is clearly demonstrated by the strong inverse relationship between τcl and biomass drought ratio (Fig. 7d). For example, the line Y4211, which showed a τcl of ~2 min, its decrease of biomass under DS was only ~20%; this is in great contrast with line T4174, which showed a τcl of ~2 min of ~8.5 min and had a decrease of biomass by ~70% under DS (Figs 6, 7d). Though a positive correlation between τcl and biomass is apparent in our used 12 accessions, we cannot eliminate the possibility that the observed positive correlation between τcl and biomass is caused by other factors, such as changed root : shoot ratio or different construction cost for the biomass.
Torres et al. (2013) conducted a large scale drought tolerance using rice genebank germplasm for yield and found that many drought tolerant accessions belong to AUS or IND group. However, three accessions out of the 12 accessions used in 2014 belong to the AUS group, but in general they showed relatively low stomatal closing speed (Table 1). We emphasise here that although faster stomatal closing is shown here to be associated with drought tolerance, rapid stomatal closing is not the only mechanism that plant use to cope with drought. The AUS and IND groups used by Torres et al. (2013) may have other drought escape, recovery, and avoidance mechanisms to gain high drought tolerance. This points to a future direction of improving drought tolerance in AUS or IND cultivars, i.e. the stomatal response dynamics can be optimised in the AUS or IND rice cultivars to gain even higher drought tolerance.
In summary, this study demonstrated that there are large natural variations in τcl and τop in response to fluctuating light in one single species. We also noted the tendency of stomatal dynamics to optimise either carbon uptake or water conservation depending on water availability and temperature of origin of the rice accession. Our findings revealed that the biomass accumulation had a positive correlation with stomatal closing speed, even though this speed was positively correlated with drought resistance. All these findings suggest that stomatal movement has a complex influence on the fitness of plants in the field and its application in rice breeding needs to consider the severity of the water stress.
Acknowledgments
We acknowledge Xianbin Yu, Shi Sun, Gupo Li, and Xianfeng Zhao for giving excellent technique assistance in large-scale photosynthetic measurements. Financial support was provided by the Chinese Academy of Sciences Strategic Leading Project ‘Designer Breeding by Molecular Module’ (grant number XDA08020301) and Project funded by 56th China Postdoctoral Science Foundation (grant number Y446C21501).
References
Agrama HA, Yan WG, Lee F, Fjellstrom R, Chen MH, Jia M, McClung A (2009) Genetic assessment of a mini-core subset developed from the USDA Rice Genebank. Crop Science 49, 1336–1346.| Genetic assessment of a mini-core subset developed from the USDA Rice Genebank.Crossref | GoogleScholarGoogle Scholar |
Amtmann R (1985) Data acquisition system for wind induced tree vibration. In ‘The forest–atmosphere interaction’. (Ed. BA Hutchinson, BB Hicks) pp. 149–159. (Reidel: Dordrecht, The Netherlands)
Cardon ZG, Berry JA, Woodrow IE (1994) Dependence of the extent and direction of average stomatal response in Zea mays L. and Phaseolus vulgaris L. on the frequency of fluctuations in environmental stimuli. Plant Physiology 105, 1007–1013.
Chazdon RL (1988) Sunflecks and their importance to forest understory plants. Advances in Ecological Research 18, 1–63.
| Sunflecks and their importance to forest understory plants.Crossref | GoogleScholarGoogle Scholar |
Dietzel L, Bräutigam K, Steiner S, Schüffler K, Lepetit B, Grimm B, Schöttler MA, Pfannschmidt T (2011) Photosystem II supercomplex remodeling serves as an entry bechanism for state transitions in Arabidopsis. The Plant Cell 23, 2964–2977.
| Photosystem II supercomplex remodeling serves as an entry bechanism for state transitions in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlSqsLzP&md5=7960a0cc874e216c1d282bb4edbbc40eCAS | 21880991PubMed |
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water use efficiency of wheat genotypes. Australian Journal of Plant Physiology 11, 539–552.
| Isotopic composition of plant carbon correlates with water use efficiency of wheat genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFSju7w%3D&md5=3c3a8b38a8403055e87bdf9712593c16CAS |
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. In ‘Annual review of plant physiology and plant molecular biology. Vol. 40’. (Ed. WR Briggs) pp. 503–538. (Annual Reviews Inc., Palo Alto, CA, USA)
Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE (2013) Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza). Plant Physiology 162, 1632–1651.
| Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFCmtLbE&md5=f6e614bccc4938012371752a63d85f95CAS | 23669746PubMed |
Kaiser H, Kappen L (1997) In situ observations of stomatal movements in different light–dark regimes: the influence of endogenous rhythmicity and long-term adjustments. Journal of Experimental Botany 48, 1583–1589.
| In situ observations of stomatal movements in different light–dark regimes: the influence of endogenous rhythmicity and long-term adjustments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmsVCrs7k%3D&md5=c5610f51b797241611b51661a7a3283fCAS |
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420, 829–832.
| Chloroplast avoidance movement reduces photodamage in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XpsFygt7o%3D&md5=0df75a24e953e6665bd06974b05478c1CAS | 12490952PubMed |
Kirschbaum MUF, Gross LJ, Pearcy RW (1988) Observed and modelled stomatal responses to dynamic light environments in the shade plant Alocasia macrorrhiza. Plant, Cell and Environment 11, 111–121.
Knapp AK, Smith WK (1988) Effect of water stress on stomatal and photosynthetic responses in Subalpine plants to cloud patterns. America Journal of Botany 75, 851–858.
| Effect of water stress on stomatal and photosynthetic responses in Subalpine plants to cloud patterns.Crossref | GoogleScholarGoogle Scholar |
Lawson T (2009) Guard cell photosynthesis and stomatal function. New Phytologist 181, 13–34.
| Guard cell photosynthesis and stomatal function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlaktrY%3D&md5=0b0010a0999ad5da7d5bb7b18d725e7dCAS | 19076715PubMed |
Lawson T, Blatt MR (2014) Stomatal size, rapidity, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiology 164, 1556–1570.
| Stomatal size, rapidity, and responsiveness impact on photosynthesis and water use efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVyqu7s%3D&md5=92efb24ac726c93403283b5a4bcc66fcCAS | 24578506PubMed |
Lawson T, Oxborough K, Morison JIL, Baker NR (2003) The response of guard and mesophyll cell photosynthesis to CO2, O2, light and water stress in a range of species are similar. Journal of Experimental Botany 54, 1743–1752.
| The response of guard and mesophyll cell photosynthesis to CO2, O2, light and water stress in a range of species are similar.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvVSmsLk%3D&md5=3789e424dd459fea046e50e6232961d0CAS | 12773521PubMed |
Lawson T, Kramer DM, Raines CA (2012) Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Current Opinion in Biotechnology 23, 215–220.
| Improving yield by exploiting mechanisms underlying natural variation of photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFGqurg%3D&md5=426eb1ba75b173f047ae1c330e371270CAS | 22296828PubMed |
Ledieu J, De Ridder P, De Clerck P, Dautrebande S (1986) A method of measuring soil moisture by time-domain reflectometry. Journal of Hydrology 88, 319–328.
| A method of measuring soil moisture by time-domain reflectometry.Crossref | GoogleScholarGoogle Scholar |
Li XB, Yan WG, Agrama H, Hu BL, Jia LM, Jia M, Jackson A, Moldenhauer K, McClung A, Wu DX (2010) Genotypic and phenotypic characterisation of genetic differentiation and diversity in the USDA rice mini-core collection. Genetica 138, 1221–1230.
| Genotypic and phenotypic characterisation of genetic differentiation and diversity in the USDA rice mini-core collection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsF2htbvN&md5=c79b3adf427a50245faa5c44e3eda8bdCAS |
Myneni RB, Impens I (1985) A procedural approach for studying the radiation regime of infinite and truncated foliage spaces. II. Experimental results and discussion. Agricultural and Forest Meteorology 33, 323–337.
| A procedural approach for studying the radiation regime of infinite and truncated foliage spaces. II. Experimental results and discussion.Crossref | GoogleScholarGoogle Scholar |
Naumburg E, Ellsworth DS, Katul GG (2001) Modeling dynamic understory photosynthesis of contrasting species in ambient and elevated carbon dioxide. Oecologia 126, 487–499.
| Modeling dynamic understory photosynthesis of contrasting species in ambient and elevated carbon dioxide.Crossref | GoogleScholarGoogle Scholar |
Ooba M, Takahashi H (2003) Effect of asymmetric stomatal response on gas-exchange dynamics. Ecological Modelling 164, 65–82.
| Effect of asymmetric stomatal response on gas-exchange dynamics.Crossref | GoogleScholarGoogle Scholar |
Ort DR (2001) When there is too much light. Plant Physiology 125, 29–32.
| When there is too much light.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjslymt70%3D&md5=b4f9af23fba56fe8bcf349fed30ef4cbCAS | 11154289PubMed |
Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annual Review of Plant Physiology 41, 421–453.
| Sunflecks and photosynthesis in plant canopies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXksFGku78%3D&md5=2008c3a59ca96e7c06be9c22f8895f59CAS |
Pfitsch WA, Pearcy RW (1989) Daily carbon gain by Adenocaulon bicolor, a redwood forest understory herb, in relation to its light environment. Oecologia 80, 465–470.
| Daily carbon gain by Adenocaulon bicolor, a redwood forest understory herb, in relation to its light environment.Crossref | GoogleScholarGoogle Scholar |
Song QF, Zhang GL, Zhu X-G (2013) Optimal crop canopy architecture to maximise canopy photosynthetic CO2 uptake under elevated CO2 – a theoretical study using a mechanistic model of canopy photosynthesis. Functional Plant Biology 40, 108–124.
| Optimal crop canopy architecture to maximise canopy photosynthetic CO2 uptake under elevated CO2 – a theoretical study using a mechanistic model of canopy photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXis1Knu74%3D&md5=bb4fd2852ca76a5ee377cb94b9778228CAS |
Stitt M, Zhu XG (2014) The large pools of metabolites involved in intercellular metabolite shuttles in C4 photosynthesis provide enormous flexibility and robustness in a fluctuating light environment. Plant, Cell and Environment 37, 1985–1988.
| The large pools of metabolites involved in intercellular metabolite shuttles in C4 photosynthesis provide enormous flexibility and robustness in a fluctuating light environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht1ChsLzM&md5=e652cc300067557549b4d28fbe2a1a56CAS | 24506493PubMed |
Torres RO, McNally KL, Cruz CV, Serraj R, Henry A (2013) Screening of rice Genebank germplasm for yield and selection of new drought tolerance donors. Field Crops Research 147, 12–22.
| Screening of rice Genebank germplasm for yield and selection of new drought tolerance donors.Crossref | GoogleScholarGoogle Scholar |
Tuberosa R, Salvi S (2006) Genomics-based approaches to improve drought tolerance of crops. Trends in Plant Science 11, 405–412.
| Genomics-based approaches to improve drought tolerance of crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xns1KjurY%3D&md5=a98a657bd352664df3496839297910ecCAS | 16843036PubMed |
Vialet-Chabrand S, Dreyer E, Brendel O (2013) Performance of a new dynamic model for predicting diurnal time courses of stomatal conductance at the leaf level. Plant, Cell and Environment 36, 1529–1546.
| Performance of a new dynamic model for predicting diurnal time courses of stomatal conductance at the leaf level.Crossref | GoogleScholarGoogle Scholar | 23448751PubMed |
Vico G, Manzoni S, Palmroth S, Katul G (2011) Effects of stomatal delays on the economics of leaf gas exchange under intermittent light regimes. New Phytologist 192, 640–652.
| Effects of stomatal delays on the economics of leaf gas exchange under intermittent light regimes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFWhsr7F&md5=b89cc345d8bd297ea3f27875eb5a646bCAS | 21851359PubMed |
Way D, Pearcy RW (2012) Sunflecks in trees and forests: from photosynthetic physiology to global change biology. Tree Physiology 32, 1066–1081.
| Sunflecks in trees and forests: from photosynthetic physiology to global change biology.Crossref | GoogleScholarGoogle Scholar | 22887371PubMed |
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VJ, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428, 821–827.
| The worldwide leaf economics spectrum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjt1Crt74%3D&md5=d60b0f91594517529da250089e292021CAS | 15103368PubMed |
Zhu XG, Ort DR, Whitmarsh J, Long SP (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. Journal of Experimental Botany 55, 1167–1175.
| The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksVagsrk%3D&md5=091144f3c71e0eddcf905436fe35495fCAS | 15133059PubMed |
Zipperlen SW, Press MC (1997) Photosynthetic induction and stomatal oscillations in relation to the light environment of two dipterocarp rain forest tree species. Journal of Ecology 85, 491–503.
| Photosynthetic induction and stomatal oscillations in relation to the light environment of two dipterocarp rain forest tree species.Crossref | GoogleScholarGoogle Scholar |






