Root anatomical plasticity contributes to the different adaptive responses of two Phragmites species to water-deficit and low-oxygen conditions
Takaki Yamauchi A * , Kurumi Sumi B , Hiromitsu Morishita B and Yasuyuki Nomura C
A * , Kurumi Sumi B , Hiromitsu Morishita B and Yasuyuki Nomura C
A
B
C
Abstract
The runner reed (Phragmites japonica) is the dominant species on riverbanks, whereas the common reed (Phragmites australis) thrives in continuously flooded areas. Here, we aimed to identify the key root anatomical traits that determine the different adaptative responses of the two Phragmites species to water-deficit and low-oxygen conditions. Growth measurements revealed that P. japonica tolerated high osmotic conditions, whereas P. australis preferred low-oxygen conditions. Root anatomical analysis revealed that the ratios of the cortex to stele area and aerenchyma (gas space) to cortex area in both species increased under low-oxygen conditions. However, a higher ratio of cortex to stele area in P. australis resulted in a higher ratio of aerenchyma to stele, which includes xylem vessels that are essential for water and nutrient uptakes. In contrast, a lower ratio of cortex to stele area in P. japonica could be advantageous for efficient water uptake under high-osmotic conditions. In addition to the ratio of root tissue areas, rigid outer apoplastic barriers composed of a suberised exodermis may contribute to the adaptation of P. japonica and P. australis to water-deficit and low-oxygen conditions, respectively. Our results suggested that root anatomical plasticity is essential for plants to adapt and respond to different soil moisture levels.
Keywords: aerenchyma, cortex, exodermis, Phragmites, root anatomical plasticity, suberin, wild Poaceae species, xylem.
Introduction
Global climate change increases the frequency and severity of single or even sequential abiotic stresses (Verslues et al. 2023); thus, it is important to understand not only how plants adapt to single environmental factors but also how they respond to various abiotic stresses. Drought and flooding are contrasting abiotic stressors that have serious impacts on agricultural production worldwide (Bailey-Serres et al. 2012; Voesenek and Bailey-Serres 2015; FAO 2021). Drought and flooding can be regarded as the deficit and excess of water in the soil, respectively. Previously, we have demonstrated that the ratio of the root tissue area of wild species of the family Poaceae is closely associated with the soil water content of their natural habitats (Yamauchi et al. 2021a). Given that soil moisture levels under natural conditions depend on precipitation levels, wild plants should possess root phenotypic plasticity to adapt to the fluctuating soil moisture levels.
Roots have three concentric cell layers: (1) the epidermis; (2) cortex; and (3) stele (Petricka et al. 2012; Tanaka et al. 2023). The cortex includes endodermis, and hypodermis and/or exodermis, and in some cases, the sclerenchyma that has secondary wall layers (Lux et al. 2004; Yamauchi et al. 2021b). Suberin and lignin depositions in the exodermis and/or sclerenchyma function as barriers to radial oxygen loss (ROL) and radial water loss under low-oxygen and water-deficit conditions, respectively (Peralta Ogorek et al. 2023). Although the primary roots of Arabidopsis thaliana have a few cortical cell layers (Petricka et al. 2012), most plant species have several cortical cell layers (Justin and Armstrong 1987). The stele transports water and nutrients through xylem vessels, whereas cortical cells demand water and nutrients through respiration (Kong et al. 2021). In contrast, the formation of aerenchyma (internal gas spaces) in the cortex reduces the respiratory demands of roots under drought and flooding (Armstrong 1980; Lynch 2018). Moreover, aerenchyma facilitates oxygen diffusion from the shoot to the root tips under flooding (Colmer and Voesenek 2009; Yamauchi et al. 2018; Pedersen et al. 2021). These findings suggest that there is an adaptive trade-off between water transport through the xylem vessels in the stele and oxygen supply through the aerenchyma in the cortex.
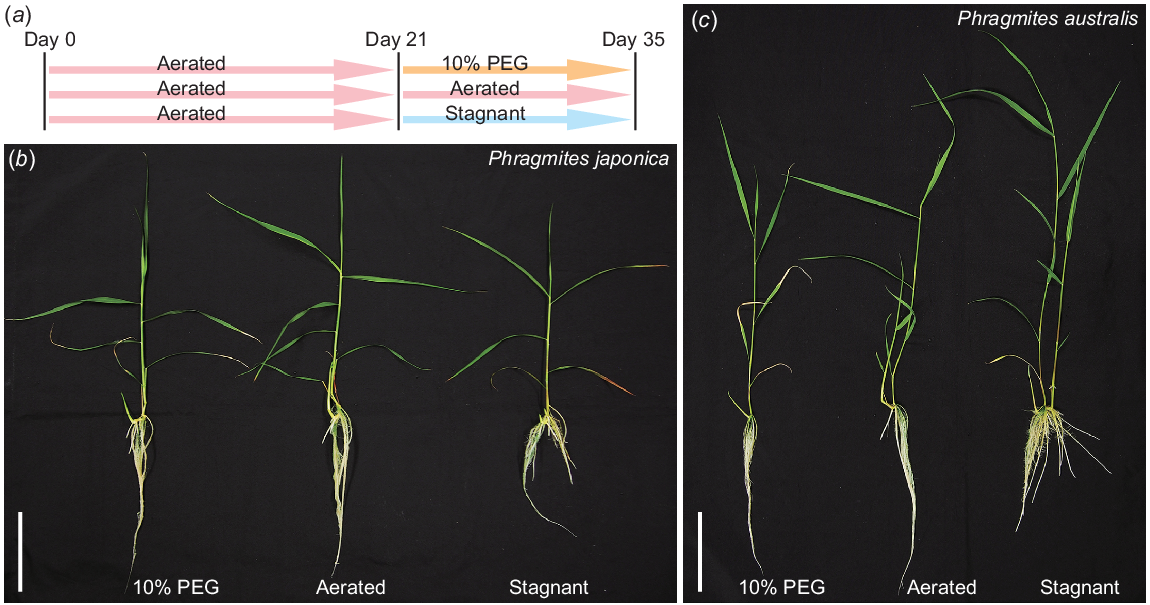
The runner reed (Phragmites japonica) is one of the dominant species found on riverbanks in East Asia (Fig. 1a; Kang et al. 2002; Asaeda et al. 2011). Field observations of the floodplain showed that the plant height and biomass of P. japonica were the lowest at sites with the lowest groundwater levels (Toda et al. 2004). As living at a site with a low groundwater level is advantageous for plants to efficiently acquire water, soil flooding could adversely affect the growth of P. japonica. In contrast, the common reed (Phragmites australis) is widely propagated by rhizomes in frequently flooded coastal marshes and the lower reaches of river and lake sediments in temperate and subtropical zones worldwide (Fig. 1b; Tulbure and Johnston 2010; Lenters et al. 2011; Rezania et al. 2019). As the leaf size, stomatal conductance and CO2 assimilation of P. australis are strongly reduced under water-deficit conditions (Pagter et al. 2005), the plant prefers flooding conditions.
Natural habitats of two Phragmites species. (a) P. japonica is found in riverbanks, where the water is never stagnant by the continuous river flows. They form stolons to escape from flooded soils. (b) P. australis is found in ponds, lakesides and lower reaches of rivers, where the water is constantly stagnant with slow water flows. They form rhizomes to stretch deep into flooded soils.

The rhizome system of P. australis is ventilated by pressurised throughflows via humidity-induced and Venturi-induced convection (Armstrong et al. 1996). Humidity-induced convection depends on the difference in humidity between the dry air outside and the humid gases in the plants (Armstrong and Armstrong 1990), whereas Venturi-induced convection is created by wind blowing across the tops of dead culms, where the wind creates suction pressure from the dead culms to the rhizome (Armstrong et al. 1992). The anatomical traits of the adventitious roots of P. australis that contribute to flood adaptation have been intensively studied. Using a microelectrode, Armstrong et al. (2000) showed that the aerenchymatous cortex of P. australis maintained a higher oxygen partial pressure than the rhizosphere (oxygen-depleted agar) because of the formation of a barrier to ROL. Moreover, the narrowing of the stele of P. australis alleviates oxygen deprivation in the inner core of the stele under oxygen-depleted agar conditions (Armstrong et al. 2000). A multiseriate exodermis with suberin lamellae and Casparian bands restricts the penetration of apoplastic tracers (Soukup et al. 2002, 2007). The suberised exodermis of P. australis supposedly contributes to ROL barrier formation (Armstrong and Armstrong 2001; Soukup et al. 2007). However, root anatomical traits of P. japonica remain largely unknown.
In this study, we aimed to identify the key root anatomical traits that determine the adaptability of plants to water-deficit and low-oxygen conditions by comparing P. japonica and P. australis, which differ in their preference for soil water levels. Therefore, we measured the growth and root anatomical traits of these two Phragmites species under high-osmotic and low-oxygen conditions and tested the genotype, treatment and genotype × treatment effects. To identify the key root anatomical traits that contribute to the growth of the two Phragmites species, correlations between growth parameters and root anatomical traits were comprehensively analysed. Moreover, histochemical staining of lignin and suberin was conducted to provide further insight into the contrasting responses of the two Phragmites species to water-deficit and low-oxygen conditions. Based on these results, we discuss the key root anatomical traits that contribute to crop improvement in order to overcome continuous or intermittent drought and/or flooding caused by ongoing climate change.
Materials and methods
Plant materials and growth conditions
Mature panicles of Phragmites japonica Steud. and Phragmites australis (Cav.) Trin. ex Steud. were placed in a plastic container with distilled water containing the fungicide (0.1% w/v benlate; Sumitomo Chemical Garden Products Inc., Tokyo, Japan), and placed in a growth chamber at 28°C under continuous light conditions (photosynthetically active radiation, 200–250 mol m−2 s−1). After 7 days, the germinated seeds were placed on mesh floats in 8-L containers containing an aerated quarter-strength nutrient solution. After 2 weeks, 21-day-old seedlings were transferred to sponges floating on the 5-L pots (eight plants per pot, 250 mm height × 120 mm length × 180 mm width) containing an aerated full-strength nutrient solution (aerated conditions), aerated high osmotic solution, or stagnant deoxygenated solution. The high-osmotic solution includes 10% (w/v) polyethylene glycol (PEG; PEG6000; Wako Pure Chemical Industries, Osaka, Japan) in aerated full-strength nutrient solution (PEG conditions). The stagnant deoxygenated solution contained 0.1% (w/v) dissolved agar (Wako Pure Chemical Industries) and was deoxygenated (dissolved oxygen < 0.5 mg L−1) before use by flushing with nitrogen gas (stagnant conditions; Wiengweera et al. 1997). The composition of the full-strength nutrient solution was as described by Colmer et al. (2006). The nutrient solutions were renewed on day 28, and the plants were further grown for 7 days. The treatments lasted for 14 days, from Days 21 to 35.
Growth measurement
Seedlings of P. japonica and P. australis were harvested at 14 days after they were transferred to aerated, PEG or stagnant conditions (35 days old). Lengths of shoots and longest adventitious roots were measured using a ruler, and the numbers of emerged leaves and adventitious roots were counted. After the measurements, Phragmites seedlings were divided into shoots and roots and dried at 60°C for 7 days in a dryer, and subsequently, the shoot and root dry weights were measured.
Root anatomical observation
Root cross-sections were prepared from 4-mm long segments excised from 80- to 120-mm long adventitious roots of P. japonica and P. australis seedlings. Root segments were prepared at 10 mm from the shoot-root junctions. Root cross-sections were prepared by hand sectioning using a razor blade. Each section was photographed using an optical microscope (ECLIPSE E600, CCD camera; DS-Ri1; Nikon, Tokyo, Japan). The area of each root tissue was measured using the ImageJ software (ver. 1.54f; National Institutes of Health, Bethesda, MD, USA) and the ratio (proportion) of each root tissue was calculated using the cross-sectional areas.
Histochemical staining of lignin and suberin
Four-millimetre long segments at 10 mm (±2 mm) from the shoot-root junctions were excised from 80- to 120-mm long adventitious roots of P. japonica and P. australis seedlings. Then 50-μm sections were made using a manual rotary microtome (Plant Microtome MTH-1, Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan). The root cross sections were stained for several minutes with saturated phloroglucinol in 20% hydrochloric acid at room temperature to visualise lignin with cinnamyl aldehyde groups in the cell walls (Jensen 1962). Each section was photographed using an optical microscope (ECLIPSE E600; CCD camera, DS-Ri1; Nikon). The root cross sections were stained with 0.01% (w/v) fluorol yellow 088 in PEG at 50°C for 1 h and washed several times with boiled water to visualise the suberin lamellae in the cell walls (Brundrett et al. 1991). The aliphatic component of suberin was detected as yellow fluorescence upon excitation with UV light (UV filter set; UV1-A, excitation filter; Ex 365/10, dichroic mirror; DM-400, barrier filter; BA-400, ECLIPSE E600; CCD camera; DS-Ri1, Nikon).
Statistical analyses
We built linear models (LMs) for the continuous variables (shoot and longest root lengths and dry weights, root tissue areas and ratios) and generalised linear models (GLMs) with Poisson distribution and log link function for the discrete variables (leaf and root numbers) to test the relationships among the different genotypes and treatments. Likelihood ratio tests (LRT) were performed to examine the significance of fixed effects. A post hoc comparison using Tukey’s honest significant difference (HSD) test was performed following the LMs and GLMs to determine the effects of the genotypes and treatments. All analyses were performed using the R software (ver. 4.3.1). The LRT was performed using the ANOVA function in the ‘car’ package (Fox and Weisberg 2011). Tukey’s HSD test was performed using the package ‘multcomp’ (Hothorn et al. 2008).
The correlation analyses among shoot and longest root lengths, leaf and root numbers, shoot and root dry weights and the ratio of root tissue areas, followed by the construction of a heatmap of the calculated correlation coefficients were performed using the ‘corrplot’ package (Wei and Simko 2017) in the R software (ver. 4.3.1). To focus on the plasticity of these parameters in response to different growth conditions, the values in the aerated conditions for each species were set at 1.0 and the relative values of each parameter were used for the analyses.
Results
Growths of two Phragmites species under high-osmotic and low-oxygen conditions
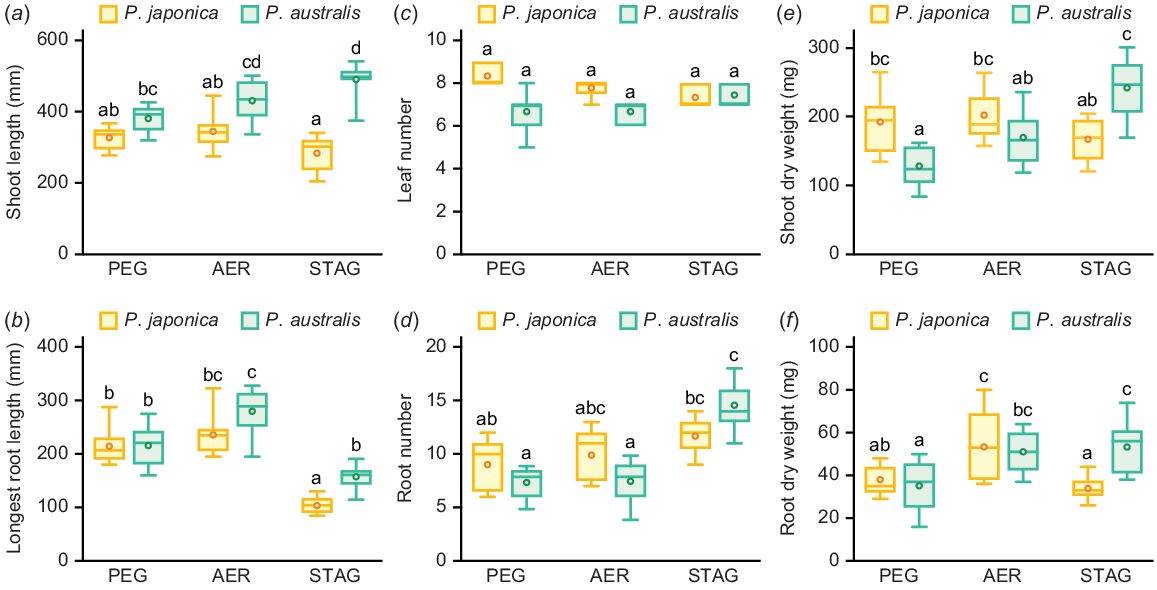
To evaluate the growth responses of P. japonica and P. australis to high-osmotic and low-oxygen conditions, 21-day-old aerobically grown seedlings were transferred to the 10% PEG (PEG), aerated (AER) and stagnant deoxygenated (STAG) conditions, which mimic water-deficit, well-drained and flooded soils, respectively (Fig. 2a). The growth of the two Phragmites species was measured 14 days after the start of the treatments (Fig. 2b, c). The shoot length of P. japonica was comparable among all three conditions, whereas the longest root length was reduced in STAG (Fig. 3a, b). The shoot length of P. australis in STAG was higher than that in PEG, whereas the longest root length of P. australis was lower in both PEG and STAG than in AER (Fig. 3a, b). No significant differences were detected in the leaf number of Phragmites species among the three conditions (Fig. 3c). The adventitious root number of P. japonica was comparable among all three conditions, whereas that of P. australis was significantly increased in STAG (Fig. 3d). Interestingly, the shoot dry weights of both Phragmites species showed similar patterns as the root numbers (Fig. 3e). The root dry weight of P. japonica was the greatest in AER, whereas the root dry weight of P. australis was greater in AER and STAG than in PEG (Fig. 3f).
Growths of two Phragmites species after the treatments under 10% PEG, aerated and stagnant conditions. (a) The seedlings of P. japonica and P. australis were grown under aerated conditions for 21 days, and then further grown under 10% PEG, aerated or stagnant conditions for 14 days, which simulate drought, drained or flooded soils. Growths of P. japonica (b) and P. australis (c) 14 days after the treatments. Bar, 10 cm.
Growth parameters of two Phragmites species 14 days after the treatments under 10% PEG (PEG), aerated (AER) and stagnant (STAG) conditions. Shoot lengths (a), longest root lengths (b), leaf numbers (c), root numbers (d), shoot dry weights (e) and root dry weights (f) of P. japonica and P. australis. Different lowercase letters above the boxplots indicate significant differences among the genotypes and treatments (P < 0.05, linear model (a, b, e, f) or generalised linear model (c, d) and Tukey’s test for multiple comparisons; n = 9). Boxplots show the median (horizontal lines), 25th to 75th percentiles (edges of the boxes), minimum to maximum (edges of the whiskers) and mean values (dots in the boxes).
Genotype × treatment interaction for growth parameters of two Phragmites species
To evaluate the interaction between the effects of genotypes and treatments on growth parameters, we built LMs for the shoot and longest root lengths and dry weights and GLMs for leaf and root numbers with log link functions (Table 1). For shoot and longest root lengths, the genotype, treatment and genotype × treatment effects were significant (Table 1). There were no significant genotype, treatment or genotype × treatment effects on leaf numbers, whereas genotype and genotype × treatment effects were detected for root numbers (Table 1). Genotype effects were not significant for shoot and root dry weights, whereas the treatment and genotype × treatment effects were significant (Table 1). Given that shoot dry weight is the most reliable parameter for evaluating the growth responses of plants to stressed conditions, the preferences for growth conditions differed between P. japonica and P. australis; that is, the P. japonica and of P. australis preferred well-drained and low-oxygen conditions, respectively (Table 1, Fig. 3e, f).
| Response variable | Model | ANOVA | Genotype | Treatment | Genotype × treatment | |
|---|---|---|---|---|---|---|
| Shoot length | LM | Pr(>F) | 1.750e-12** | 0.04597* | 1.289e-05*** | |
| Root length | LM | Pr(>F) | 0.0005679** | 6.031e-15** | 0.04702* | |
| Leaf number | GLM | Pr(>|t|) | 0.2288 | 0.95330000 | 0.60670000 | |
| Root number | GLM | Pr(>|t|) | 0.63560000 | 2.677e-06** | 0.02610* | |
| Shoot dry weight | LM | Pr(>F) | 0.4688 | 0.002932* | 1.839e-06*** | |
| Root dry weight | LM | Pr(>F) | 0.1183 | 3.799e-04** | 0.004288** |
LM, linear model; GLM, generalised linear model.
*P < 0.05; **P < 0.01; ***P < 0.001.
Root anatomical traits of two Phragmites species under high osmotic and low-oxygen conditions
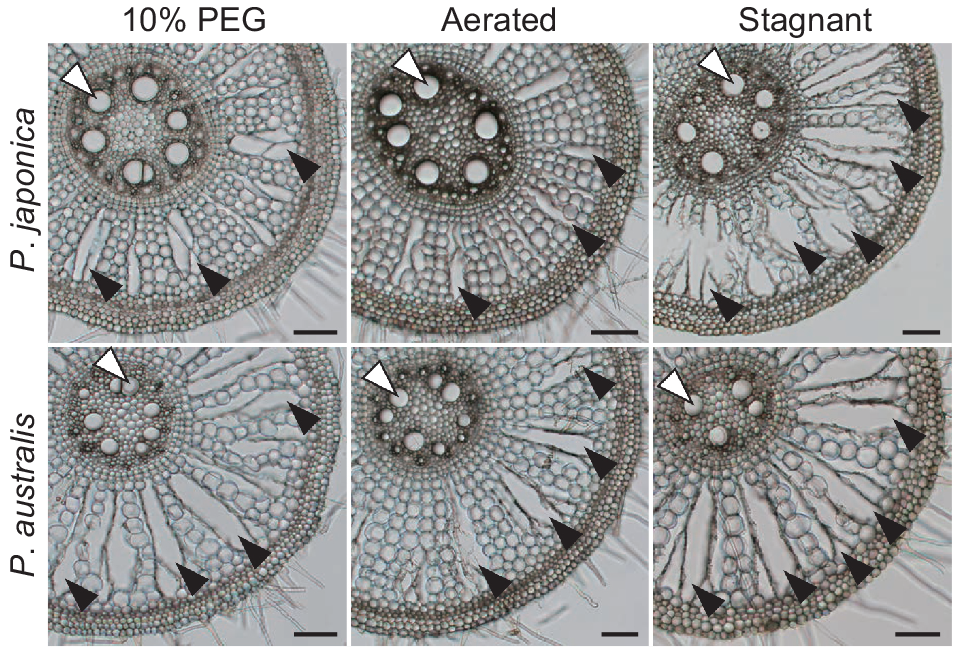
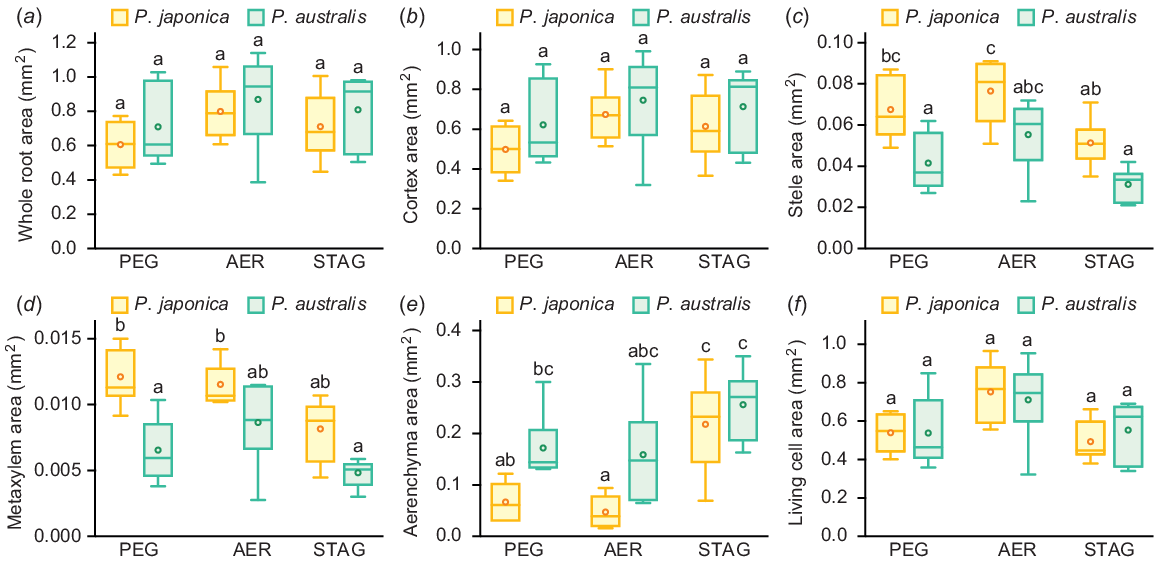
To compare the root anatomical traits of the two Phragmites species, root cross-sections at 10 mm from the shoot-root junctions were prepared from 80- to 120-mm long adventitious roots of P. japonica and P. australis grown in PEG, AER and STAG for 14 days (Fig. 4). Root cross-sectional areas (whole root areas) and cortical areas were comparable among the three conditions (Fig. 5a, b). The stele area of P. japonica was significantly decreased in STAG, whereas that of P. australis was comparable among all three conditions (Fig. 5c). The areas of late metaxylems were comparable among the three conditions (Fig. 5d). The aerenchyma area of P. japonica significantly increased in STAG, whereas that of P. australis was comparable among the three conditions (Fig. 5e). Living cell areas (root cross-sectional areas – aerenchyma areas) were comparable under all three conditions (Fig. 5f). Taken together, the root tissue areas were strongly affected by the variation in root cross-sectional areas, and this variation masked the clear patterns of root anatomical plasticity.
The root cross sections at 10 mm from the shoot-root junctions of adventitious roots of two Phragmites species 14 days after the treatments under 10% PEG (PEG), aerated (AER) and stagnant (STAG) conditions. Lysigenous aerenchyma and large metaxylems are indicated by black and white arrowheads, respectively. Bar, 100 μm.
The areas of root tissues of two Phragmites species 14 days after the treatments under 10% PEG (PEG), aerated (AER) and stagnant (STAG) conditions. Root cross-sectional area (whole root area) (a), and the areas of cortex (b), stele (c), large metaxylem (d), aerenchyma (e) and living cell (root cross-sectional area – aerenchyma area) (f) at 10 mm from the shoot-root junctions of P. japonica and P. australis. Different lowercase letters above the boxplots indicate significant differences among the genotypes and treatments (P < 0.05, linear model and Tukey’s test for multiple comparisons) (n = 6). Boxplots show the median (horizontal lines), 25th to 75th percentiles (edges of the boxes), minimum to maximum (edges of the whiskers) and mean values (dots in the boxes).
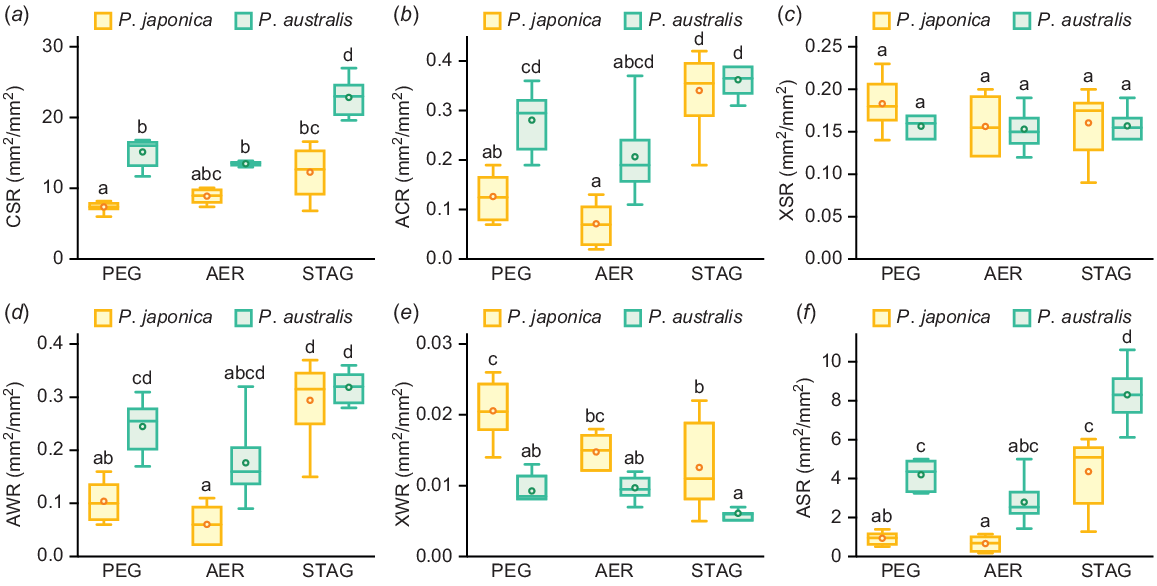
Previously, we demonstrated that the ratio of root tissue areas in the basal parts of adventitious roots of wild species of the family Poaceae was closely associated with soil moisture levels in their natural habitats (Yamauchi et al. 2021a). Thus, we calculated the ratio of each root tissue area at 10 mm from the shoot-root junctions (Fig. 6). The adaptive significance of each root tissue ratio is summarised in Table 2. The cortex to stele ratio (CSR) of both Phragmites species was comparable between PEG and AER, and that of P. australis significantly increased in STAG (Fig. 6a). Although the difference between the AER and PEG or STAG was not significant, the CSR of P. japonica in STAG was higher than that in PEG (Fig. 6a). The aerenchyma to cortex ratio (ACR) of P. japonica was significantly increased in STAG, whereas that of P. australis was comparable among all three conditions (Fig. 6b). The xylem to stele ratio (XSR) was comparable among all three conditions (Fig. 6c). The patterns of the aerenchyma to whole root ratio (AWR) were similar to those of ACR (Fig. 6d). The xylem to whole root ratio (XWR) of P. japonica decreased in STAG when compared with that of PEG (Fig. 6e). The aerenchyma to stele ratio (ASR) of both Phragmites species was comparable between PEG and AER; however, it significantly increased in STAG (Fig. 6f). Interestingly, the CSR and ASR of P. australis in PEG and STAG were significantly higher than those of P. japonica (Fig. 6a, f).
The ratio of root tissue areas of two Phragmites species 14 days after the treatments under 10% PEG (PEG), aerated (AER) and stagnant (STAG) conditions. The ratio of the areas of cortex to stele (CSR) (a), aerenchyma to cortex (ACR) (b), xylem to stele (XSR) (c), aerenchyma to whole root (AWR) (d), xylem to whole root (XWR) (e) and aerenchyma to stele (ASR) (f) at 10 mm from the shoot-root junctions of P. japonica and P. australis. Different lowercase letters above the boxplots indicate significant differences among the genotypes and treatments (P < 0.05, linear model and Tukey’s test for multiple comparisons) (n = 6). Boxplots show the median (horizontal lines), 25th to 75th percentiles (edges of the boxes), minimum to maximum (edges of the whiskers) and mean values (dots in the boxes).
| Root | Abbreviation | Adaptive significance | |
|---|---|---|---|
| Cortex to stele ratio | CSR | CSR represents the balance of cortex and stele proportion in roots. | |
| High CSR and low CSR are associated with large aerenchyma and xylem vessels, and are advantageous for adaptation to flooding and drought, respectively. | |||
| Aerenchyma to cortex ratio | ACR | ACR indicaties the efficiency of aerenchyma formation in the cortex. | |
| A high ACR is advantageous for adaptation to both drought and flooding, owing to the reduction in energy costs for the maintenance of cortical cells. | |||
| Xylem to stele ratio | XSR | XSR indicates the efficiency of xylem vessel formation in the stele. | |
| High XSR may contribute to efficient water and nutrient uptake, owing to the increase in xylem areas and numbers. | |||
| Aerenchyma to whole root ratio | AWR | AWR indicates the efficiency of aerenchyma formation in roots. | |
| The AWR is affected by the CSR and ACR. High CSR and ACR can result in very high AWR, which strongly contributes to efficient oxygen diffusion in the roots. | |||
| Xylem to whole root ratio | XWR | XWR indicates the efficiency of xylem vessel formation in roots. | |
| The XWR is affected by the CSR and XSR. Low CSR and high XSR can result in very high XWR, which strongly contributes to efficient root water uptake. | |||
| Aerenchyma to stele ratio | ASR | ASR represents the balance of aerenchyma and stele proportions in roots. | |
| A high ASR is advantageous for adaptation to flooding, as a large amount of aerenchyma supports the oxygen demand in a small stele. |
Genotype × treatment interaction of root anatomical traits in two Phragmites species
To further analyse the interaction between the effects of genotype and treatment on root anatomical traits (ratio of each root tissue), we built LMs for CSR, ACR, XSR, AWR, XWR and ASR (Table 3). The genotype, treatment and genotype × treatment effects were significant for CSR, ACR and AWR (Table 3). No significant genotype, treatment or genotype × treatment effects for XSR were found; however, genotype and treatment effects were detected for XWR and ASR (Table 3). The significant effects of genotype × treatment on CSR, ACR and AWR suggest that the plasticity of the cortex proportion, which is associated with the proportion of aerenchyma in the roots, is a key determinant of the different adaptive responses of P. japonica and P. australis.
| Response variable | Model | ANOVA | Genotype | Treatment | Genotype × treatment | |
|---|---|---|---|---|---|---|
| CSR | LM | Pr(>F) | 4.263e-12*** | 7.459e-09*** | 0.005649** | |
| ACR | LM | Pr(>F) | 2.502e-05*** | 9.805e-09*** | 0.03055* | |
| XSR | LM | Pr(>F) | 0.2585 | 0.4247 | 0.5314 | |
| AWR | LM | Pr(>F) | 1.518e-05*** | 6.904e-09*** | 0.03466* | |
| XWR | LM | Pr(>F) | 1.834e-07*** | 0.001098** | 0.1051 | |
| ASR | LM | Pr(>F) | 3.756e-09*** | 7.996e-11*** | 0.1597 |
LM, linear model.
*P < 0.05; **P < 0.01; ***P < 0.001.
Correlation of growth parameters and root anatomical traits in two Phragmites species
To identify the key root anatomical traits that determine the adaptive responses of the two Phragmites species, correlation analyses were performed for growth parameters and the ratio of root tissue area (Fig. 7). To focus on plasticity under different growth conditions, the values in AER of each species were set at 1.0 and the relative values were subjected to Pearson’s correlation analyses. Shoot lengths (SL), leaf numbers (LN) and shoot dry weights (SDW) were strongly positively correlated (r > 0.79; Fig. 7a). Longest root lengths (RL) negatively correlated with root numbers (RN, r = −0.61); however, they did not correlate with root dry weights (RDW, r = 0.37; Fig. 7a). A weak positive correlation was detected between root numbers (RN) and root dry weights (RDW, r = 0.45; Fig. 7a). A strong positive correlation was detected between shoot dry weights (SDW) and root numbers (RN, r = 0.85) and root dry weights (RDW, r = 0.76), indicating that shoot and root growth interacted with each other (Fig. 7a).
Correlation analyses of the growth parameters and ratio of root tissue areas of two Phragmites species 14 days after the treatments under 10% PEG (PEG), aerated (AER) and stagnant (STAG) conditions. To focus on the responses of the growth parameters and ratio of root tissue areas to the conditions in PEG and STAG, the value of each parameter in AER was set as 1.0, and the relative values were used for Pearson correlation analyses. The heatmap of the correlations among shoot and root growth parameters (a), shoot growth parameters with the ratio of root tissue areas (b) and root growth parameters with the ratio of root tissue areas (c) were shown. SL, shoot length; LN, leaf number; SDW, shoot dry weight; RL, root length; RN, root number; RDW, root dry weight; CSR, cortex to stele ratio; ACR, aerenchyma to cortex ratio; XSR, xylem to stele ratio.
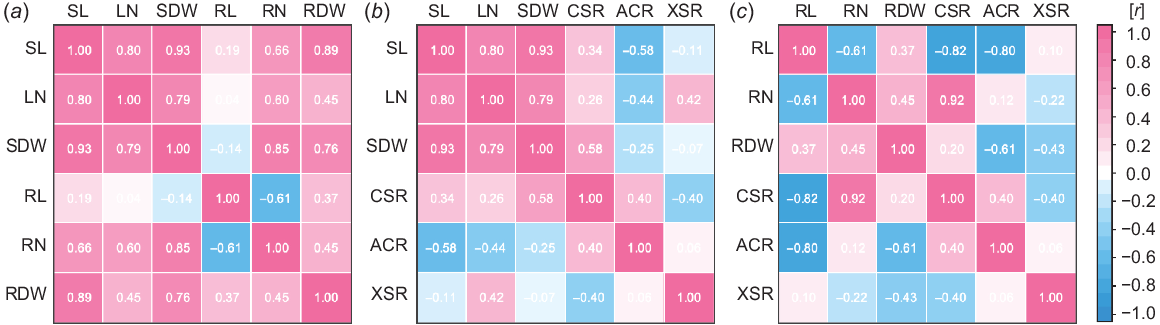
The root anatomical traits CSR, ACR and XSR (Table 2) showed low correlation with each other (−0.4 < r < 0.4; Fig. 7b), suggesting that these parameters have different response patterns. The correlations between root anatomical traits and shoot growth parameters (−0.58 < r < 0.58; Fig. 7b) were weaker than those between root anatomical traits and root growth parameters (−0.82 < r < 0.92; Fig. 7c). However, relatively strong positive and negative correlations were detected between CSR and SDW (r = 0.58), and between ACR and SL (r = −0.58; Fig. 7b). Interestingly, CSR showed strong positive and negative correlations with RN (r = 0.92) and RL (r = −0.82), respectively (Fig. 7b). ACR was negatively correlated with RL (r = −0.8) and RDW (r = −0.61), whereas XSR showed a weak negative correlation with RDW (r = −0.43; Fig. 7b). These results were due to the fact that aerenchyma formation was high in PEG and STAG, whereas the shoot growth was negatively affected by high-osmotic and low-oxygen stresses, except for P. australis in STAG (Figs 3e and 6b). In contrast, the plastic responses of CSR and RN to high-osmotic and low-oxygen stresses were always associated with the shoot growth responses (Figs 3e and 6a).
Deposition of lignin and suberin in the outer part of the roots in two Phragmites species
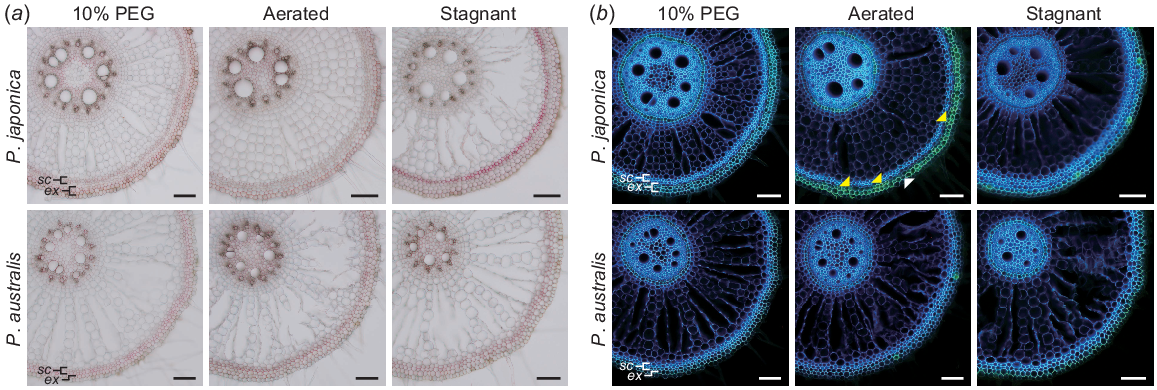
Lignin and suberin deposition 10 mm from the shoot-root junctions of the adventitious roots of the two Phragmites species were detected using phloroglucinol-HCl and fluorol yellow 088, respectively (Fig. 8). Two layers of sclerenchyma and exodermis in the roots of P. japonica and two layers of sclerenchyma and a single layer of exodermis in the roots of P. australis were identified (Fig. 8). Lignification was detected in the exodermis of P. japonica under all conditions; it was faint in AER, increased in PEG and further increased in STAG (Fig. 8a). In contrast, lignification was detected to some extent in the exodermis and sclerenchyma of P. australis under all conditions (Fig. 8a). Strong suberisation was detected in the outer exodermal cells of P. japonica; however, suberin deposition in the AER was patchy in the inner exodermal cell layers, and the outer exodermis had non-suberised passage cells (Fig. 8b). Interestingly, both exodermal cell layers of P. japonica were fully suberised in PEG, whereas suberisation of the exodermis was faint in STAG (Fig. 8b). In contrast, a fully suberised exodermis was detected in P. australis in both PEG and STAG (Fig. 8b).
The deposition of lignin and suberine in the outer part of adventitious roots at 10 mm from the shoot-root junctions of two Phragmites species 14 days after the treatments under 10% PEG, aerated and stagnant conditions. (a), Lignin deposition was detected as red staining with phloroglucinol-HCl. (b), Suberin lamellae was detected as yellow fluorescence with fluorol yellow 088 under UV irradiation. A passage cell in the outer exodermis and the suberised inner exodermis of P. japonica in aerated conditions are indicated by white and yellow arrowheads, respectively. sc, sclerenchyma; ex, exodermis. Bar, 100 μm.
Discussion
Growth assessments under PEG (aerated conditions with 10% PEG), AER (aerated conditions) and STAG (stagnant deoxygenated conditions), which mimic water-deficit, well-drained and flooded soils, respectively, revealed that shoot and root dry weights of P. japonica were greatest in AER, whereas those of P. australis were greatest in STAG (Figs 2 and 3e, f). These results well fitted to their habitat preferences under natural conditions: P. japonica is found on riverbanks where water is never stagnant, and P. australis is found in ponds, lakes and the lower reaches of the river where water is constantly stagnant (Fig. 1a, b; Toda et al. 2004; Pagter et al. 2005). The shoot and longest root lengths of P. japonica did not significantly decrease in PEG (Fig. 3a, b), indicating that P. japonica has a higher tolerance to water-deficit conditions than P. australis. In contrast, all growth parameters except for the longest root length of P. australis were greatest in the STAG (Fig. 3), suggesting that P. australis adapts to low-oxygen conditions under flooding. The significant genotype × treatment effects on shoot and longest root lengths, and shoot and root dry weights further support the idea that the adaptive responses of P. japonica and P. australis are different (Table 1).
The responses of the root anatomical traits to high-osmotic and low-oxygen conditions differed between P. japonica and P. australis (Fig. 4). Because the root tissue areas showed high variability among the roots (Fig. 5), we used the ratio of root tissue areas, which had strong correlations with soil moisture levels in the natural habitats of wild species of the family Poaceae (Yamauchi et al. 2021a; Fig. 6). The ratios of the cortex area to the stele area (CSR) and the aerenchyma area to the cortex area (ACR) showed similar patterns in the two Phragmites species (Fig. 6a, b). Higher CSR and ACR in STAG support the idea that a higher proportion of cortex and aerenchyma, which contribute to efficient oxygen diffusion within roots (Armstrong 1980; Colmer 2003a), is advantageous for adapting to low-oxygen conditions. Although ACR in STAG was comparable between the two Phragmites species, higher CSR in P. australis resulted in a much higher ratio of the aerenchyma area to the stele area (ASR; Fig. 6f), which implies that the greater amount of aerenchyma supports the oxygen demand in the stele of P. australis. Because anoxia in the stele restricts the loading of essential ions into xylem vessels (Colmer and Greenway 2011; Kotula et al. 2015; Armstrong et al. 2019), P. australis having a larger cortex and aerenchyma and a smaller stele possibly would render it more adaptive to low-oxygen conditions. In contrast, a lower CSR in P. japonica grown in PEG might contribute to the tolerance of P. japonica to water-deficit conditions, as a high stele proportion is associated with a high xylem proportion in the root cross-sectional area (Fig. 6a, e), despite large xylem not always being beneficial for drought tolerance concerning the water use efficiency under water-limited field conditions (Richards and Passioura 1989; Yamauchi et al. 2021b). Moreover, a low CSR is potentially associated with a small number of cortical cell files, which contributes to efficient water uptake in dry soils (Chimungu et al. 2014). The significant genotype × treatment effects for CSR and ACR suggest that the plasticity of the cortex and aerenchyma proportions are key for the responses to water-deficit and low-oxygen conditions (Table 3).
Aerenchyma formation, as indicated by ACR, was detected in P. japonica and P. australis even in the AER (Fig. 6b), indicating that both Phragmites species constitutively formed aerenchyma, even under aerobic conditions. Lysigenous aerenchyma formation in roots can be classified into two types: constitutive aerenchyma formation, which is detected in the roots of wetland species under well-drained aerobic conditions, and inducible aerenchyma formation, which is detected in the roots of both wetland and upland species in response to low-oxygen conditions under flooding (Colmer and Voesenek 2009). Indeed, rice (Oryza sativa) and a wild wetland relative of maize constitutively form aerenchyma, whereas wheat (Triticum aestivum) and maize (Zea mays) hardly form aerenchyma under aerobic conditions (Yamauchi et al. 2019, 2020; Ning et al. 2023). Because constitutive aerenchyma formation was more pronounced in the roots of P. australis than in those of P. japonica (Fig. 6b), P. australis was more adaptive to low-oxygen conditions. A similar observation was made in the natural habitats of two ecotypes of cogongrass (Imperata cylindrica), where the wetland ecotype formed greater aerenchyma than the dryland ecotype, even at moderate soil moisture levels (Nomura et al. 2024). These interspecies variations may be useful for understanding the mechanisms underlying constitutive and inducible aerenchyma formation, which are regulated by different mechanisms (Yamauchi and Nakazono 2022a, 2022b).
Interestingly, aerenchyma formation was also detected in the roots of P. japonica and P. australis in PEG (Fig. 6b). Root aerenchyma formation reduces the respiratory demands of the roots; thus, its formation is beneficial for the deep rooting and plant growth under drought (Zhu et al. 2010; Lynch 2018). However, a high amount of aerenchyma in P. australis did not positively affect its growth under PEG (Figs 3e and 6b). In our previous studies, the ACR values of dryland species were comparable to those of wetland species (Yamauchi et al. 2021a). As aerenchyma formation in both Phragmites species was not significantly increased by the PEG treatment (Fig. 6b), (constitutive) aerenchyma formation in both Phragmites species could be involved in adaptation to flooding. Nevertheless, the possibility that artificial water-deficit (high-osmotic) conditions are unsuitable for evaluating the contribution of aerenchyma formation to root growth cannot be excluded, as the longest root lengths of both Phragmites species did not increase in PEG (Fig. 3b).
Correlation analyses of growth parameters and the ratio of root tissue areas in the two Phragmites species revealed that shoot dry weights showed the strongest positive correlations with root numbers (RN) and CSR among the three root growth parameters and anatomical indices (Fig. 7a, b). Interestingly, CSR and RN also showed a strong positive correlation (Fig. 7b), indicating that they are important root traits for adaptative responses to water-deficit and low-oxygen conditions. Although larger cortex and aerenchyma proportions are beneficial for efficient oxygen diffusion into the root tips through the aerenchyma (Pedersen et al. 2021), smaller stele and xylem proportions may adversely affect plant growth owing to the reduction of water and nutrient uptake from the soils (Yamauchi et al. 2021b). Indeed, large aboveground parts are associated with thick roots, large stele and xylem vessels (Wahl and Ryser 2000). The most effective way to overcome this situation is to increase the number of roots, which compensates for the less effective water and nutrient uptake from individual roots (Yamauchi et al. 2021b). Rice has a much larger number of adventitious roots than wheat and maize, which have larger stele and xylem vessels (Yamauchi et al. 2019). In contrast, large stele and xylem vessels are associated with deep rooting under water-deficit conditions (Price et al. 2002; Uga et al. 2008). The reduction in RN may support the deep rooting the whole plant or whole root economic spectrum (Kong et al. 2019; Iversen and McCormack 2021).
Although substantial aerenchyma formation was detected in the basal part of P. japonica, its longest root length was strongly reduced in STAG (Figs 3b and 6b). A similar finding was obtained in wheat and maize, which form more than half of the aerenchyma in the roots of rice in STAG (Yamauchi et al. 2019). A barrier to ROL from the aerenchyma to the rhizosphere is an essential adaptive trait for plants under soil flooding, as its formation aids efficient oxygen diffusion from the shoot to the root tips and impedes the influx of toxic substances from the rhizosphere (Colmer 2003a; Ejiri et al. 2021; Mano and Nakazono 2021; Pedersen et al. 2021). Indeed, P. australis has been used as a sort of model plant for wetland species with a tight ROL barrier in its roots (Armstrong et al. 1992, 2000). As the patterns of ROL barrier formation vary among species, ecotypes and varieties (Colmer 2003b; Ejiri and Shiono 2019; Ejiri et al. 2020; Tong et al. 2023), the strong reduction in the longest root length of P. japonica might be caused by its weak or no ROL barrier formation. A study using oxygen microelectrodes showed that the exodermal cell layer, where suberisation is detected (Soukup et al. 2007), is responsible for a greater part of the decrease in oxygen partial pressure in an oxygen-depleted agar (Armstrong et al. 2000). Indeed, suberisation in the exodermis was induced in the roots of P. australis in STAG (Fig. 8b). In contrast, suberisation in the exodermis of P. japonica rather decreased in STAG (Fig. 8b), suggesting that P. japonica does not form a tight barrier to ROL in the outer part of the roots. Interestingly, P. japonica has two layers of fully suberised exodermis in PEG (Fig. 8b). These suberised exodermal cell layers might contribute to the radial diffusion resistance to water vapour, which restricts the radial water loss from the root surfaces under high-osmotic conditions (Peralta Ogorek et al. 2021; Song et al. 2023).
Conclusion
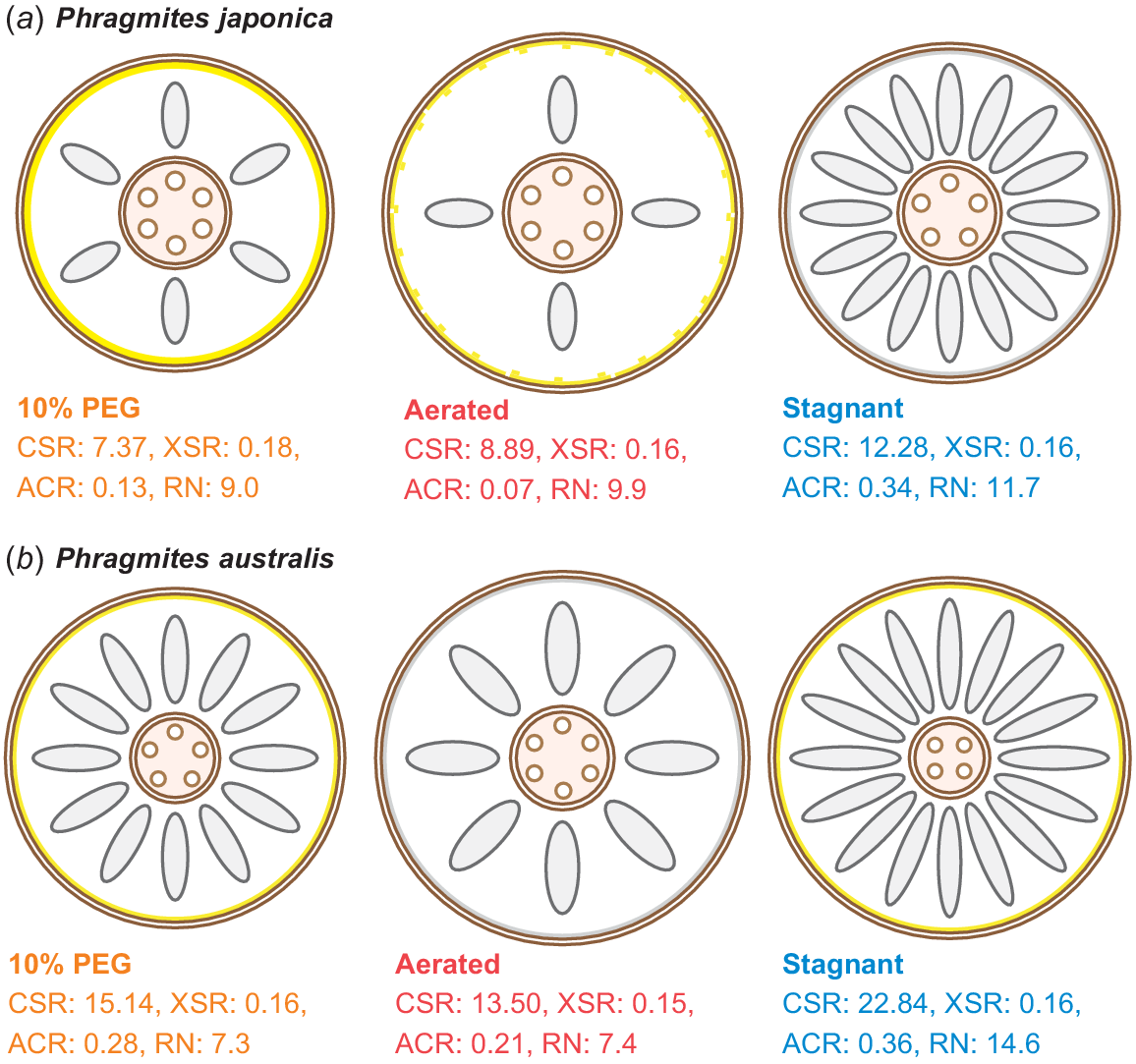
In this study, we identified the root anatomical traits that contribute to the different adaptive responses of the two Phragmites species to water-deficit and low-oxygen conditions (Fig. 9). The roots of P. japonica had larger stele and xylem proportions (lower CSR and higher XSR) than the roots of P. australis and formed aerenchyma under high-osmotic conditions (Fig. 9a). Although P. australis forms more aerenchyma (higher ACR) than P. japonica, the proportions of stele and xylem areas were smaller (higher CSR and lower XSR) under high-osmotic conditions (Fig. 9a, b). Moreover, the two layers of suberised exodermis in the roots of P. japonica may prevent radial water loss more efficiently than P. australis, which has only a single layer of suberised exodermis (Fig. 9a, b). In contrast, P. australis having a much larger proportion of cortex and aerenchyma (higher CSR and ACR) than P. japonica, was more adaptive to low-oxygen (stagnant) conditions (Fig. 9b). A smaller proportion of stele (higher CSR) may adversely affect the water and nutrient uptake in the roots of P. australis; however, a larger root number (higher RN) can compensate for this disadvantage (Fig. 9b). Moreover, the suberised exodermis of P. australis may contribute to the formation of a barrier to ROL under low-oxygen conditions (Fig. 9b), whereas the growth reduction in P. japonica under low-oxygen conditions may be at least partly caused by the absence of a fully suberised exodermis (Fig. 9a). Although further studies are needed to confirm whether functional barriers against water vapour and oxygen exist in roots of the two Phragmites species, root anatomical plasticity will remain a key aspect for understanding plant adaptation to drought and flooding, and for crop improvement to overcome continuous or intermittent drought and/or flooding stress caused by ongoing climate change.
Schematic diagrams of plasticity in root anatomical traits of two Phragmites species in 10% PEG, aerated and stagnant conditions. The mean values of the ratio of cortex to stele area (CSR), xylem to stele area (XSR) and aerenchyma to cortex area (ACR) at the basal part of the adventitious roots were used to draw the root anatomy of P. japonica (a) and P. australis (b). The means of large metaxylem numbers of P. japonica were 5.83, 6.17 and 5.00, and those of P. australis were 5.33, 5.83 and 3.83 in 10% PEG, aerated and stagnant conditions, respectively. The root numbers (RNs) positively correlated to the values of CSR (Fig. 7c). Suberin lamellae in the exodermis is indicated by yellow lines. Suberisation was patchy in the exodermis of P. japonica in aerated conditions (Fig. 8b).
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This work was partly supported by a Grant-in-Aid for Transformative Research Areas (A), MEXT KAKENHI, Grant Numbers, 23H04195; Japan Science and Technology Agency PRESTO Grants, JPMJPR17Q8 to T.Y.; and JSPS KAKENHI (Grant Number JP21K14955) to Y.N.
Acknowledgements
The authors thank Timothy D. Colmer, Ole Pedersen and Mikio Nakazono for stimulating discussions and Nobuhiro Tsutsumi for encouragement. The authors also thank Tsutomu Yamaguchi (ESPEC MIC Corp.) for providing the seeds of Phragmites species and valuable information on their adaptation.
References
Armstrong W (1980) Aeration in higher plants. Advances in Botanical Research 7, 225-332.
| Crossref | Google Scholar |
Armstrong J, Armstrong W (1990) Light-enhanced convective throughflow increases oxygenation in rhizomes and rhizosphere of Phragmites australis (Cav.) Trin. ex Steud. New Phytologist 114, 121-128.
| Crossref | Google Scholar | PubMed |
Armstrong J, Armstrong W (2001) Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. American Journal of Botany 88, 1359-1370.
| Crossref | Google Scholar |
Armstrong J, Armstrong W, Beckett PM (1992) Phragmites australis: Venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytologist 120, 197-207.
| Crossref | Google Scholar |
Armstrong J, Armstrong W, Beckett PM, Halder JE, Lythe S, Holt R, Sinclair A (1996) Pathways of aeration and the mechanisms and beneficial effects of humidity- and Venturi-induced convections in Phragmites australis (Cav.) Trin. ex Steud. Aquatic Botany 54, 177-197.
| Crossref | Google Scholar |
Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM (2000) Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Annals of Botany 86, 687-703.
| Crossref | Google Scholar |
Armstrong W, Beckett PM, Colmer TD, Setter TL, Greenway H (2019) Tolerance of roots to low oxygen: ‘anoxic’ cores, the phytoglobin-nitric oxide cycle, and energy or oxygen sensing. Journal of Plant Physiology 239, 92-108.
| Crossref | Google Scholar | PubMed |
Asaeda T, Baniya MB, Rashid MH (2011) Effect of floods on the growth of Phragmites japonica on the sediment bar of regulated rivers: a modelling approach. International Journal of River Basin Management 9, 211-220.
| Crossref | Google Scholar |
Bailey-Serres J, Lee SC, Brinton E (2012) Waterproofing crops: effective flooding survival strategies. Plant Physiology 160, 1698-1709.
| Crossref | Google Scholar | PubMed |
Brundrett MC, Kendrick B, Peterson CA (1991) Efficient lipid staining in plant material with sudan red 7B or fluoral yellow 088 in polyethylene glycol-glycerol. Biotechnic & Histochemistry 66, 111-116.
| Crossref | Google Scholar | PubMed |
Chimungu JG, Brown KM, Lynch JP (2014) Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiology 166, 1943-1955.
| Crossref | Google Scholar | PubMed |
Colmer TD (2003a) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26, 17-36.
| Crossref | Google Scholar |
Colmer TD (2003b) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany 91, 301-309.
| Crossref | Google Scholar | PubMed |
Colmer TD, Greenway H (2011) Ion transport in seminal and adventitious roots of cereals during O2 deficiency. Journal of Experimental Botany 62, 39-57.
| Crossref | Google Scholar | PubMed |
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36, 665-681.
| Crossref | Google Scholar | PubMed |
Colmer TD, Cox MCH, Voesenek LACJ (2006) Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytologist 170, 767-778.
| Crossref | Google Scholar | PubMed |
Ejiri M, Shiono K (2019) Prevention of radial oxygen loss is associated with exodermal suberin along adventitious roots of annual wild species of Echinochloa. Frontiers in Plant Science 10, 254.
| Crossref | Google Scholar |
Ejiri M, Sawazaki Y, Shiono K (2020) Some accessions of Amazonian wild rice (Oryza glumaepatula) constitutively form a barrier to radial oxygen loss along adventitious roots under aerated conditions. Plants 9, 880.
| Crossref | Google Scholar | PubMed |
Ejiri M, Fukao T, Miyashita T, Shiono K (2021) A barrier to radial oxygen loss helps the root system cope with waterlogging-induced hypoxia. Breeding Science 71, 40-50.
| Crossref | Google Scholar | PubMed |
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical Journal 50, 346-363.
| Crossref | Google Scholar | PubMed |
Iversen CM, McCormack ML (2021) Filling gaps in our understanding of belowground plant traits across the world: an introduction to a Virtual Issue. New Phytologist 231, 2097-2103.
| Crossref | Google Scholar | PubMed |
Justin SHFW, Armstrong W (1987) The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106, 465-495.
| Crossref | Google Scholar |
Kang S, Kang H, Ko D, Lee D (2002) Nitrogen removal from a riverine wetland: a field survey and simulation study of Phragmites japonica. Ecological Engineering 18, 467-475.
| Crossref | Google Scholar |
Kong D, Wang J, Wu H, Valverde-Barrantes OJ, Wang R, Zeng H, Kardol P, Zhang H, Feng Y (2019) Nonlinearity of root trait relationships and the root economics spectrum. Nature Communications 10, 2203.
| Crossref | Google Scholar | PubMed |
Kong D, Wang J, Valverde-Barrantes OJ, Kardol P (2021) A framework to assess the carbon supply-consumption balance in plant roots. New Phytologist 229, 659-664.
| Crossref | Google Scholar | PubMed |
Kotula L, Clode PL, Striker GG, Pedersen O, Läuchli A, Shabala S, Colmer TD (2015) Oxygen deficiency and salinity affect cell-specific ion concentrations in adventitious roots of barley (Hordeum vulgare). New Phytologist 208, 1114-1125.
| Crossref | Google Scholar | PubMed |
Lenters JD, Cutrell GJ, Istanbulluoglu E, Scott DT, Herrman KS, Irmak A, Eisenhauer DE (2011) Seasonal energy and water balance of a Phragmites australis-dominated wetland in the Republican River basin of south-central Nebraska (USA). Journal of Hydrology 408, 19-34.
| Crossref | Google Scholar |
Lux A, Luxová M, Abe J, Morita S (2004) Root cortex: structural and functional variability and responses to environmental stress. Root Research 13, 117-131.
| Crossref | Google Scholar |
Lynch JP (2018) Rightsizing root phenotypes for drought resistance. Journal of Experimental Botany 69, 3279-3292.
| Crossref | Google Scholar | PubMed |
Mano Y, Nakazono M (2021) Genetic regulation of root traits for soil flooding tolerance in genus Zea. Breeding Science 71, 30-39.
| Crossref | Google Scholar | PubMed |
Ning J, Yamauchi T, Takahashi H, Omori F, Mano Y, Nakazono M (2023) Asymmetric auxin distribution establishes a contrasting pattern of aerenchyma formation in the nodal roots of Zea nicaraguensis during gravistimulation. Frontiers in Plant Science 14, 1133009.
| Crossref | Google Scholar | PubMed |
Nomura Y, Arima S, Kyogoku D, Yamauchi T, Tominaga T (2024) Strong plastic responses in aerenchyma formation in F1 hybrids of Imperata cylindrica under different soil moisture conditions. Plant Biology
| Crossref | Google Scholar |
Pagter M, Bragato C, Brix H (2005) Tolerance and physiological responses of Phragmites australis to water deficit. Aquatic Botany 81, 285-299.
| Crossref | Google Scholar |
Pedersen O, Sauter M, Colmer TD, Nakazono M (2021) Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytologist 229, 42-49.
| Crossref | Google Scholar | PubMed |
Peralta Ogorek LL, Pellegrini E, Pedersen O (2021) Novel functions of the root barrier to radial oxygen loss – radial diffusion resistance to H2 and water vapour. New Phytologist 231, 1365-1376.
| Crossref | Google Scholar | PubMed |
Peralta Ogorek LL, Jiménez JdlC, Visser EJW, Takahashi H, Nakazono M, Shabala S, Pedersen O (2023) Outer apoplastic barriers in roots: prospects for abiotic stress tolerance. Functional Plant Biology 51, FP23133.
| Crossref | Google Scholar |
Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Annual Review of Plant Biology 63, 563-590.
| Crossref | Google Scholar | PubMed |
Price AH, Cairns JE, Horton P, Jones HG, Griffiths H (2002) Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. Journal of Experimental Botany 53, 989-1004.
| Crossref | Google Scholar | PubMed |
Rezania S, Park J, Rupani PF, Darajeh N, Xu X, Shahrokhishahraki R (2019) Phytoremediation potential and control of Phragmites australis as a green phytomass: an overview. Environmental Science and Pollution Research 26, 7428-7441.
| Crossref | Google Scholar | PubMed |
Richards RA, Passioura JB (1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research 40, 943-950.
| Crossref | Google Scholar |
Song Z, Zonta F, Ogorek LLP, Bastegaard VK, Herzog M, Pellegrini E, Pedersen O (2023) The quantitative importance of key root traits for radial water loss under low water potential. Plant and Soil 482, 567-584.
| Crossref | Google Scholar |
Soukup A, Votrubová O, Čížková H (2002) Development of anatomical structure of roots of Phragmites australis. New Phytologist 153, 277-287.
| Crossref | Google Scholar |
Soukup A, Armstrong W, Schreiber L, Franke R, Votrubová O (2007) Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytologist 173, 264-278.
| Crossref | Google Scholar | PubMed |
Tanaka W, Yamauchi T, Tsuda K (2023) Genetic basis controlling rice plant architecture and its modification for breeding. Breeding Science 73, 3-45.
| Crossref | Google Scholar | PubMed |
Toda Y, Hashido N, Ikeda S (2004) Study on growth and nutrient uptake of Phragmites japonica on flood plain in a gravel river. Proceedings of Hydraulic Engineering 48, 1615-1620.
| Crossref | Google Scholar |
Tong S, Kjær JE, Peralta Ogorek LL, Pellegrini E, Song Z, Pedersen O, Herzog M (2023) Responses of key root traits in the genus Oryza to soil flooding mimicked by stagnant, deoxygenated nutrient solution. Journal of Experimental Botany 74, 2112-2126.
| Crossref | Google Scholar | PubMed |
Tulbure MG, Johnston CA (2010) Environmental conditions promoting non-native Phragmites australis expansion in great lakes coastal wetlands. Wetlands 30, 577-587.
| Crossref | Google Scholar |
Uga Y, Okuno K, Yano M (2008) QTLs underlying natural variation in stele and xylem structures of rice root. Breeding Science 58, 7-14.
| Crossref | Google Scholar |
Verslues PE, Bailey-Serres J, Brodersen C, Buckley TN, Conti L, Christmann A, Dinneny JR, Grill E, Hayes S, Heckman RW, et al. (2023) Burning questions for a warming and changing world: 15 unknowns in plant abiotic stress. The Plant Cell 35, 67-108.
| Crossref | Google Scholar | PubMed |
Voesenek LACJ, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytologist 206, 57-73.
| Crossref | Google Scholar | PubMed |
Wahl S, Ryser P (2000) Root tissue structure is linked to ecological strategies of grasses. New Phytologist 148, 459-471.
| Crossref | Google Scholar | PubMed |
Wei T, Simko V (2017) R package “corrplot”: visualization of a correlation matrix (version 0.84). Available at https://github.com/taiyun/corrplot
Wiengweera A, Greenway H, Thomson CJ (1997) The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Annals of Botany 80, 115-123.
| Crossref | Google Scholar |
Yamauchi T, Nakazono M (2022a) Mechanisms of lysigenous aerenchyma formation under abiotic stress. Trends in Plant Science 27, 13-15.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Nakazono M (2022b) Modeling-based age-dependent analysis reveals the net patterns of ethylene-dependent and -independent aerenchyma formation in rice and maize roots. Plant Science 321, 111340.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Colmer TD, Pedersen O, Nakazono M (2018) Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiology 176, 1118-1130.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Abe F, Tsutsumi N, Nakazono M (2019) Root cortex provides a venue for gas-space formation and is essential for plant adaptation to waterlogging. Frontiers in Plant Science 10, 259.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Tanaka A, Tsutsumi N, Inukai Y, Nakazono M (2020) A role for auxin in ethylene-dependent inducible aerenchyma formation in rice roots. Plants 9, 610.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Pedersen O, Nakazono M, Tsutsumi N (2021a) Key root traits of Poaceae for adaptation to soil water gradients. New Phytologist 229, 3133-3140.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Noshita K, Tsutsumi N (2021b) Climate-smart crops: key root anatomical traits that confer flooding tolerance. Breeding Science 71, 51-61.
| Crossref | Google Scholar | PubMed |
Zhu J, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant, Cell & Environment 33, 740-749.
| Crossref | Google Scholar | PubMed |


