Non-invasive approaches for phenotyping of enhanced performance traits in bean
Uwe Rascher A D , Stephan Blossfeld A , Fabio Fiorani A , Siegfried Jahnke A , Marcus Jansen A , Arnd J. Kuhn A , Shizue Matsubara A , Lea L. A. Märtin A , Andrew Merchant B , Ralf Metzner A , Mark Müller-Linow A , Kerstin A. Nagel A , Roland Pieruschka A , Francisco Pinto A , Christina M. Schreiber A , Vicky M. Temperton A , Michael R. Thorpe A , Dagmar van Dusschoten A , Elizabeth van Volkenburgh C , Carel W. Windt A and Ulrich Schurr AA Institute of Bio- and Geosciences, IBG-2: Plant Sciences, Forschungszentrum Jülich GmbH, Leo-Brandt-Str., 52425 Jülich, Germany.
B Faculty of Agriculture, Food and Natural Resources, Biomedical Building, The University of Sydney, 1 Central Avenue, Eveleigh, NSW 2006, Australia.
C Biology Department, Box 35-5325, University of Washington, Seattle, WA 98195, USA.
D Corresponding author. Email: u.rascher@fz-juelich.de
Functional Plant Biology 38(12) 968-983 https://doi.org/10.1071/FP11164
Submitted: 26 July 2011 Accepted: 15 October 2011 Published: 1 December 2011
Abstract
Plant phenotyping is an emerging discipline in plant biology. Quantitative measurements of functional and structural traits help to better understand gene–environment interactions and support breeding for improved resource use efficiency of important crops such as bean (Phaseolus vulgaris L.). Here we provide an overview of state-of-the-art phenotyping approaches addressing three aspects of resource use efficiency in plants: belowground roots, aboveground shoots and transport/allocation processes. We demonstrate the capacity of high-precision methods to measure plant function or structural traits non-invasively, stating examples wherever possible. Ideally, high-precision methods are complemented by fast and high-throughput technologies. High-throughput phenotyping can be applied in the laboratory using automated data acquisition, as well as in the field, where imaging spectroscopy opens a new path to understand plant function non-invasively. For example, we demonstrate how magnetic resonance imaging (MRI) can resolve root structure and separate root systems under resource competition, how automated fluorescence imaging (PAM fluorometry) in combination with automated shape detection allows for high-throughput screening of photosynthetic traits and how imaging spectrometers can be used to quantify pigment concentration, sun-induced fluorescence and potentially photosynthetic quantum yield. We propose that these phenotyping techniques, combined with mechanistic knowledge on plant structure–function relationships, will open new research directions in whole-plant ecophysiology and may assist breeding for varieties with enhanced resource use efficiency varieties.
Additional keywords: fluorescence, imaging spectroscopy, non-invasive, resource use efficiency.
Phenotyping for improved agricultural resource use efficiency in the 21st century
The green revolution of the 1950s resulted in a remarkable increase in average yield of most crops (Evans 1997). For example, roughly 50% of increased corn production can be ascribed to genetic improvements and 50% to improved agricultural management practices (Duvick 2005). Key factors include progress in molecular breeding techniques that reduced the time required for developing new genotypes and the availability of relatively cheap energy justifying larger use of fertiliser and water. Today, the task to further increase yield by these mechanisms faces considerable challenges (Godfray et al. 2010). In past decades, increased energy consumption in the agricultural sector has been both economically and socially viable, with few restrictions imposed on increasing resource inputs. In today’s climate, agricultural practices must increasingly consider the trade-off of pursuing higher yield against the costs of more intensive management. Present and future plant research objectives must now address optimisation of resource use efficiency and ensure the stability of yield at the regional and global scale (see Ainsworth et al. 2011). In this respect, bean and other protein producing plants may become increasingly important in the near future to provide proteins directly for human consumption and to contribute to the increasing demand of protein-rich livestock feed in times of increasing global meat consumption. Plant phenotyping may not only provide possibilities for a total increase of protein production but may also help to direct protein quality and specific protein content.
To pursue these objectives, significant reliance is placed upon plant phenotyping capabilities. Phenotyping is rapidly evolving as a new discipline in plant sciences (see special issue of Functional Plant Biology Volume 26, Issue 10–11) using non- or minimally-invasive sensors to measure plant performance and uncover genetic determinants of enhanced agronomic traits. Complex plant physiological traits, such as growth, biomass accumulation and yield, result from the interplay of genetic and environmental determinants throughout the plant (crop) growing season. Environmental factors modulate the expression of genes in space and throughout developmental time and greatly influence the phenotype. As a result, important physiological traits vary along a continuum following a dose–response paradigm. Responses can be generalised for individual trait-environmental factor pairs using a quantitative meta-analytical framework (Poorter et al. 2010). A recent overview on the challenges of phenomics and the statistical aspects of the gene–environment interplay was presented by Houle et al. (2010).
Phenotyping can take place under laboratory, greenhouse or field conditions. Under laboratory conditions, environmental factors may be controlled and varied as desired, manipulating one or more factors in dedicated experiments. Via this controlled approach, the influence of specific genetic and chemical factors interacting with a limited number of environmental factors can be investigated. In contrast, field conditions are highly variable, fluctuating in time and space (Rascher and Nedbal 2006; Schurr et al. 2006; Mittler and Blumwald 2010). Despite this, new technologies capable of high-throughput phenotyping coupled with environmental monitoring at high spatial and temporal resolution can deliver datasets large enough for statistical approaches. For example, imaging spectroscopy can provide high resolution pictures from ground and airborne platforms that contain high resolution spectral data of millions of pixels (Rascher et al. 2009). Single spectra can be attributed to single plants or experimental plots and related to plant functional traits (Ustin and Gamon 2010).
In this communication we present state-of-the-art technologies and approaches for plant phenotyping and how these can be applied to populate the trait matrix referring to whole-plant resource use efficiency (Table 1). Plant phenotyping and improved phenological understanding are targeted towards specific traits that influence improved resource use efficiency in plants. Below-ground traits such as root architecture or root function are associated with improved water and nitrogen use efficiency; above-ground traits are mainly linked to photosynthetic energy conversion and, thus, govern light use efficiency; transport and allocation processes link the components of plants and have a great influence on nitrogen and water use efficiency of plants (Table 1). In the following we will give a brief definition of our terms.
Light use efficiency
Above-ground processes are largely dictated by photosynthesis and the conversion of solar energy to biomass. Photosynthetic light use efficiency (LUE) is defined as the amount of fixed carbon per unit of absorbed (or incident) photons. At low light intensity net CO2 assimilation rate is linearly dependent on irradiance and LUE is maximal as well as constant. Crop productivity can be improved through enhancement of photosynthetic LUE (Long et al. 2006; Murchie et al. 2009). The strategies are divided into two non-mutually exclusive approaches: optimisation of light harvesting and optimisation of carbon assimilation. Improved light harvesting may be achieved by either modifying the biophysical and biochemical properties of photosynthesis on the leaf level (enhancing quantum efficiency of photosystem or minimising energy loss from photoprotective dissipation; (Long et al. 2006) or by modification of canopy architecture and light interception in the canopy (altered leaf angle distribution and canopy closure; Rascher et al. 2010a). Enhanced carbon assimilation may result from molecular engineering of Rubisco (Parry et al. 2003; Whitney et al. 2011), or introduction of C4 traits into C3 crops (Hibberd et al. 2008; Gowik and Westhoff 2011). Overall, the values of LUE of grain legumes are somewhat lower than in other crop plants, presumably because of relatively high energy costs required for symbiotic N fixation (Sinclair and Muchow 1999).
Water use efficiency
Water use efficiency is a complex property that depends on a multitude of above-ground, below-ground and transport processes. In agronomy, water use efficiency (WUE) is defined as the ratio between dry matter accumulation (or yield) and the total amount of water spent by the crop over the same period (Tambussi et al. 2007). At a leaf level, WUE is defined as net CO2 assimilation rate (A) divided by transpiration rate (E); WUE calculated in this way is also called ‘instantaneous’ WUE (WUEA/E). A related parameter is the ‘intrinsic’ WUE defined as the ratio between A and stomatal conductance (gs) (WUEA/gs; Osmond Björkman and Anderson 1980). Compared with WUEA/E, WUEA/gs is less influenced by vapour pressure deficit (VPD) between leaf and air, rendering WUEA/gs a better parameter to characterise genotypic differences of leaf processes (Gilbert et al. 2011). At the plant scale, root architecture and function greatly determine foraging and exploration of water resources below-ground. Recent studies point towards a high importance of root growth and angles for water uptake (Lynch and Jonathan 2007; Hammer et al. 2009) and, thus, are an important influence on whole-plant WUE.
Nitrogen use efficiency
The nitrogen use efficiency (NUE) is determined mainly by below-ground and transport and allocation processes. In agriculture, NUE is defined as a ratio of biomass production to input of nutrient fertiliser. NUE can be expressed as crop yield (biomass or crop harvest) per unit of N available in the soil (Moll et al. 1982), dry matter produced per N content in the plant (Good et al. 2004), or the proportion of N taken up and metabolised by plants as a fraction of totally available N. Due to their ability to obtain N from symbiotic fixation of atmospheric N2, legumes can accumulate large amounts of N in non-fertilised soil; for example, in well watered conditions ~65 and 85% of the total N content of cowpea and soybean plants, respectively, can be attributed to N fixation (Sinclair et al. 1987).
The main source of N changes for plants between the vegetative phase and the reproductive phase (Hirel et al. 2007). Young vegetative plants rely on N uptake from the soil or N-fixing symbionts. N remobilisation from the existing structures (e.g. Rubisco degradation) increases at later stages, generally after flowering. N fixation during seed filling varies among different species (Sinclair et al. 1987). Breeding strategies to improve NUE are, therefore, directed to optimisation of physiological or structural properties related to N uptake, assimilation or remobilisation (Hirel et al. 2007; Masclaux-Daubresse et al. 2010; Kant et al. 2011).
Optimised resource use of above-ground energy conversion
In nature, photosynthesis operates under temporally highly fluctuating conditions with the photosynthetic apparatus adapted to an ever changing and highly variable stream of photons (Rascher and Nedbal 2006). Plants compete for above-ground space to support the light reactions of photosynthesis. As a consequence, plants may invest in foliage beyond the need to capture light with plant growth driving photosynthesis, rather the other way round (Körner 2011). In intensive agriculture, however, conditions are different. Nutrient limitation is often avoided and plants are planted in monocultures with high planting densities. Under these conditions, light energy may become a limiting factor suggesting improvement of photosynthetic efficiency may be a valid longer term focus for breeders and farmers (Long et al. 2006; Zhu et al. 2010). More specifically, it has been argued that three main processes may address improvements in photosynthetic efficiency: (i) waste of energy by non-photochemical dissipation may be optimised on the cellular level (Zhu et al. 2004); (ii) the balance between transpiration and CO2 uptake may be engineered at the leaf level (with implications for plant growth under global change) (Long and Ort 2010); and (iii) the penetration and interception of light within the dense canopy of crops could be improved by optimised leaf, plant and canopy structure (Dermody et al. 2008; Rascher et al. 2010).
High-throughput screening for optimised photosynthesis by automated fluorescence techniques
Rapid and non-invasive measurements of photosynthetic parameters are essential in screening for plants with optimised photosynthetic capacity and may facilitate the selection process in plant breeding. Chlorophyll fluorescence measurements are highly attractive because they allow fast and non-invasive determination of relevant photosynthetic parameters such as quantum yield, electron transport rate, or non-photochemical quenching (Maxwell and Johnson 2000; Baker 2008).
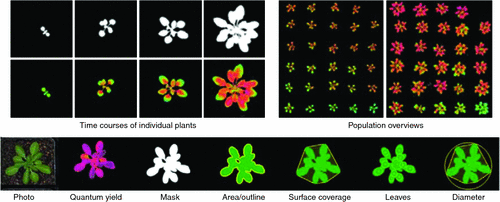
GROWSCREEN FLUORO is an automated screening device that combines a fluorescence imager (Maxi PAM, Walz GmbH, Germany) with computer controlled moving stages and automated data analyses routines for high-throughput measurement of relevant photosynthetic parameters. This set-up enables an automated screening of the model species Arabidopsis thaliana (L.) Heynh. at a throughput of 60 plants per hour (Fig. 1; Jansen et al. 2009). Moreover, projected leaf area and morphological parameters for describing the shoot architecture can be extracted automatically from the chlorophyll fluorescence images (Fig. 1).
|
Simultaneous measurement of photosynthesis, growth and morphology delivers multiple phenotypic data and helps in understanding how environmental factors modulate the plant phenotype. By acquiring time courses of phenotypic factors (for example, see Fig. 1 for growth and quantum yield), we can investigate how and when differences between plants start developing. Concomitantly, the considerable throughput of 60 plants per hour enables comparison of populations (Fig. 1), for example, for the detection of mutants that react differently to given environments. GROWSCREEN FLUORO analyses can reveal influences of mutations, transgenes or environmental factors on diverse phenotypic properties of the plants. Such high-throughput phenotyping facilitates functional genomics by linking phenotypes to given genotypes to help select plants with enhanced resource use efficiency or stress tolerance. Whereas GROWSCREEN FLUORO itself was developed for the model species A. thaliana, the system can be adapted to a range of canopy architectures across a diversity of crop plants.
Mapping crops by imaging spectroscopy
Imaging spectroscopy describes a novel discipline within optical remote sensing and is driven by the rapid development in sensor technologies. Until recently, imaging techniques relied on the use of a few spectral bands. Nowadays it is technically feasible to measure a continuous spectrum of light using imaging sensors (see Ustin et al. 2004; Rascher et al. 2007). How can this technology be applied to plant tissues? In brief, solar radiation that interacts with plant tissues or plant canopies is reflected, absorbed or transmitted. Spectral characteristics of the three components at leaf or canopy scale are a function of the optical properties of leaves, stems and other canopy components, canopy architecture and effects such as observation geometry. Imaging spectroscopy focuses on the reflected part of radiation to derive information about the biochemical and structural properties of plants at leaf and canopy level. For instance, the low reflectance of plant leaves in the visible (400–700 nm) range of the light spectrum results from the strong absorbance of the photosynthetic foliar pigments, whereas the high reflectance in the near infrared (700–1100 nm) is due to low absorption of light by the internal leaf mesophyll tissues. Reflectance in the shortwave infrared (1100–2500 nm) is strongly affected by the amount of water in plant tissues (Curran 1989; Rascher et al. 2010b).
During the phenological cycle, and in response to environmental conditions, the biochemical components of plants and canopies change, resulting in a changing interaction with solar radiation. Using knowledge of the specific absorption and reflection properties of these components, one can characterise single leaf and canopy components from the reflected light. For practical reasons, vegetation sciences often concentrate on a few relevant spectral regions and often use normalised difference indices, such as the normalised difference vegetation index (NDVI) that is correlated with the chlorophyll content and leaf area index (LAI). However, methods in spectral feature extraction have greatly improved and it can be expected that advanced quantification methods will be used in plant sciences in the near future. For example, principle component analyses in combination with decision tree algorithms can be used for early detection of leaf rust (Franke et al. 2005) or inversion of leaf optical models can map invasive species (Asner and Vitousek 2005). Recently it even became feasible to extract sun-induced fluorescence from high-performance imaging spectroscopy data which will open a new path of remote sensing of plant functionality (Malenovsky et al. 2009; Meroni et al. 2009; Rascher et al. 2009).
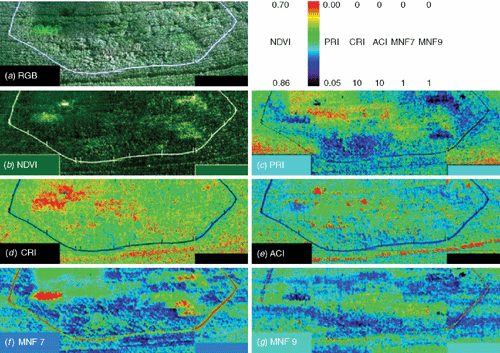
Imaging spectroscopy can be used for plant phenotyping in the field. The Soy-FACE (free-air carbon dioxide enrichment) facility provides an ideal test bed for such a case study as several varieties of soy bean cultivars are grown under elevated CO2 and O3 conditions (Rogers et al. 2004). Hyperspectral image cubes of the Soy-FACE facility of the University of Illinois, Urbana-Champaign, were acquired in July 2004 with the SOC-700 (Surface Optics Corp, San Diego, CA, USA). The instrument is a line scanner that can be operated from a wide range of distances and acquires 12 bit radiance images between 440 and 880 nm with ~4 nm spectral resolutions yielding hyperspectral cubes consisting of 120 spectral bands (for detailed description of the instrument see Rascher et al. 2007). Images were taken around mid-day from a height of ~20 m at an angle of ~45° using an elevated platform. About 10 single images were taken to cover the experimental setup. A reflectance standard (50% Spectralon, Labsphere, North Sutton, NH, USA) was positioned for each image and relative reflectances were calculated for each image. Single images were later registered to one another to provide a full image of the experiment. Reflectance images were filtered using the filtering procedure in ‘principal component space’ as described in detail by Rascher et al. (2007). From the filtered images, selected vegetation indices were calculated (formulae see legend in Fig. 2).
|
In Fig. 2, different maps were calculated from the hyperspectral reflectance data cubes. Fig. 2a shows a red, green blue (RGB) colour composite of the study site for visual orientation. Using the red and near-infrared spectrum of reflectance, the widely used NDVI can be calculated (Fig. 2b). Fig. 2c shows the photochemical reflectance index (PRI) that is reported to be related to the light use efficiency of photosynthesis (Gamon et al. 1992). Further indices are the carotenoid reflectance index (CRI) and the anthocyanin reflectance index (ACI) that measure the carotenoid and anthocianin content, respectively (Gitelson et al. 2001, 2002) (Fig. 2d, e). Figure 2f, g shows the principal component bands 7 and 9 that were calculated according to the mean noise fraction (MNF). MNF analyses rotate the spectral data cube according to their main information content and thus reveal subtle differences in the reflectance properties that are however hard to describe analytically (see Rascher et al. 2007 for details on this advanced approach).
In each map, the different variety of the soybean cultivars shows its specific properties and the cultivars can be distinguished from each other by their functional and structural characteristics. For separation, classification methods such as support vector machines can be used. Expanding this approach for different crop species is currently under way.
Mapping of structure and function of natural canopies by hyperspectral, sun-induced fluorescence and stereo imaging techniques
Canopies are complex, three dimensional (3-D) assemblages of leaves with various orientations and have an unpredictable surface structure. Light intensity and quality change quickly within natural canopies at different time scales exposing single leaf elements to a variable stream of photons (Frak et al. 2002; Yamashita et al. 2002). Dynamic acclimation of photosynthetic efficiency to these changes in environmental light conditions are still not well understood (Rascher and Nedbal 2006) and novel approaches to measure canopy structure and function simultaneously are urgently needed.
Canopy structure
Spatial and temporal distribution of leaf angles are important indicators for the canopy function and plant state. Diurnal heliotropic leaf movements, which were described for 16 different plant families (Ehleringer and Forseth 1980), contribute to high LUE by optimising light distribution within the canopy over the course of day (Kao and Forseth 1992). Furthermore, under drought stress and N limitation, paraheliotropic leaf movement (i.e. direction of leaf lamina parallel to the sun’s ray) enhances WUE and NUE (Kao and Forseth 1991). The canopy structure within plant populations is important to estimate the cultivation density avoiding, for instance, excessive neighbour competition. The separation and analysis of each of these levels as well as applications like the extraction of particular population parameters, e.g. height, LAI or the density of pods, can be addressed by further image processing of the 3-D stereos.
Over the past few years, computer stereo vision has been extensively studied in systems where 3-D information of objects in their environment is of crucial importance, e.g. in automatic processing of autonomous systems and robotics (e.g. van der Mark and Gavrila 2006; Olson et al. 2007). Taking advantage of these dramatic improvements in stereo vision, we developed a stereo approach that is specifically suited for the 3-D mapping of natural plant canopies in the field (Biskup et al. 2007, 2009). The use of two synchronised digital SLR cameras allows 3-D mapping that is robust against canopy movements and varying illumination and that permits the monitoring of plant architecture on different scales in the field. In a first case study, we were able to use this stereo system to quantify diurnal changes in leaf orientations in soybean. Highly resolved data on leaf angle distribution, together with forward modelling of sun movement and light penetration in a complex 3-D canopy, leads to a better understanding of the interplay of structural and functional properties in radiation use in natural canopies (Rascher et al. 2010a).
Canopy function
A robust approach to measure the light use efficiency of photosynthesis is the quantification of chlorophyll (Chl) a fluorescence (Maxwell and Johnson 2000; Baker 2008). However, in conventional fluorometry the photosynthetic apparatus needs to be excited actively, reducing applicability to large spatial scales under natural light. One active, scanning approach is LIFT, which was successfully used to measure fluorescence parameters and to detect stress response from a distance of 50 m (Ananyev et al. 2005; Kolber et al. 2005; Rascher and Pieruschka 2008; Pieruschka et al. 2010). Recently, the passive non-invasive detection of the sun-induced Chl fluorescence (Fs) retrieved from the spectral signature of the canopy by using the Fraunhofer line depth (FLD) principle, has become a promising approach to overcome this problem (Plascyk and Gabriel 1975; Moya et al. 2004; Liu et al. 2005; Alonso et al. 2008; Malenovsky et al. 2009; Meroni et al. 2009). There is experimental and theoretical evidence that Fs can also be correlated with photosynthetic efficiency and the stress induced limitation of photosynthetic electron transport and thus may serve as a proxy to quantify photosynthetic LUE (Rosema et al. 1998; Flexas et al. 2000; Meroni and Colombo 2006; Damm et al. 2010). It has been established, for example, that at low light, when the non-photochemical protection mechanism is not activated, there is a negative correlation between the photochemistry and the Fs signal (van der Tol et al. 2009).
Structure–function relation
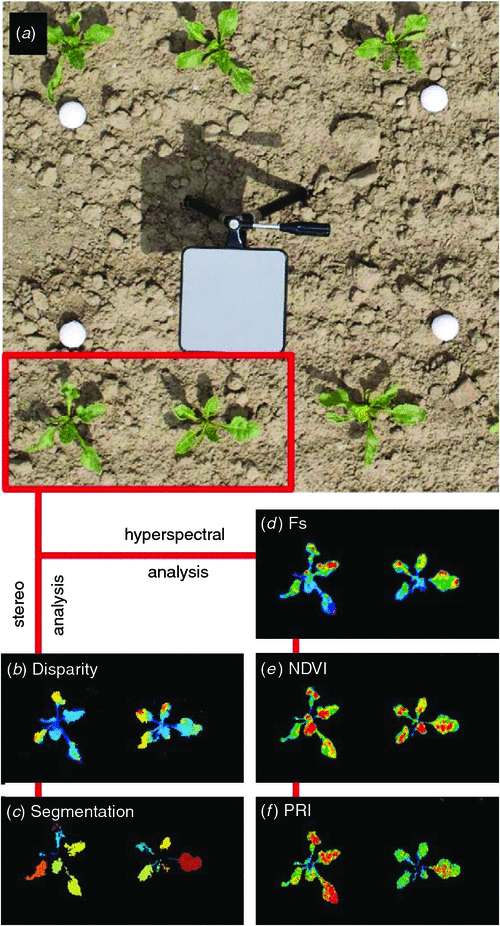
Figure 3 displays co-registered 3-D and hyperspectral images and further processing steps using an example of sugar beet (Beta vulgaris L. var. Pauletta). Two steps of processing stereo images (Fig. 3a only left stereo image shown) are depicted, including the 3-D reconstruction (Fig. 3e the disparity denotes the depth information) and the automatic selection of particular plant structures (Fig. 3f segmentation of single leaves). Further steps include the quantification of leaf angles with respect to species- and variety-specific leaf models and the derivation of light absorption properties.
|
In Fig. 4d, the spatial distribution of the sun-induced fluorescence signal (Fs) is presented. The interpretation of the Fs signal might depend not only on the physiological status of the plants, but also on the light-leaf interaction determined by the orientation of the different canopy elements. Thus, the combination of Fs with the 3-D parameterisation of this data offers a unique opportunity for a better understanding of the structural and functional changes of photosynthesis adjustments under changing light conditions within the canopy. Hyperspectral information is also used to obtain other physiological parameters commonly used in remote sensing. In Fig. 3e and 3f the NDVI and PRI are shown respectively. We are currently working to adapt these approaches to a variety of canopies and we expect that robust approaches for field phenotyping will be available soon.
|
Transport and allocation processes
Allocation, transport and communication between above- and below-ground components have long been recognised as fundamental processes underpinning plant function. Traditional approaches are largely based upon invasive sampling of plant components with little regard for direct measures of resource flux. For most agricultural crops the final product is the seed or fruit. In agronomy there are several ways to relate crop biomass or carbon budget to seed yield through the ‘harvest index’. However, increasing total biomass per se may not always result in higher final yield. It is intuitive that transport and allocation processes are a strong pre-determinant of yield.
Visualising transport and growth processes by nuclear magnetic resonance techniques
The use of nuclear magnetic resonance (NMR) to monitor plant processes non-invasively can take several forms. For example, sap flow can be measured by magnetic resonance imaging (MRI) techniques without the need to spatially resolve the conducting tissue. MRI can also be used, even without imaging, to assess non-invasively the dynamics of growth and water status. In the latter case the technique is no longer called nuclear magnetic resonance imaging (NMRI), but simply magnetic resonance (MR) or, more formally, NMR. A positive side effect of needing no, or only low resolution imaging, is, that the NMR equipment can become simpler and smaller. This even makes it possible to use portable scanner-like instrumentation, suitable for the application in the field.
Measuring sap flow using MRI velocimetry
An especially appealing application of MRI is sap flow imaging. Xylem and phloem sap flows are difficult to study because of their extreme sensitivity to invasive experimentation (Knoblauch et al. 2001). Consequently, little is known regarding the dynamics of sap flow in an intact plant. Considering the pivotal role of the xylem and phloem as all-connecting superhighways in plants’ water and carbohydrate economy (Tyree and Ewers 1991; van Bel 2003), this is surprising. MRI flow imaging is uniquely suited to fill this methodological gap. It is non-invasive and does not require contrast agents or marker fluids; it merely records the displacement of water inside the plant.
Although the use of MRI velocimetry in the botanical sciences is still in its infancy, its potential in studying transport towards fruit has already been demonstrated. Recently it was used to quantify xylem and phloem translocation towards a growing tomato truss (Windt et al. 2009). Phloem and xylem translocation in the truss stalk were measured, with the objective to quantify their relative contributions. The results were surprising. It had been commonly believed that the overwhelming majority of water reaches the fruit by means of the phloem and that the xylem becomes dysfunctional at an early stage of fruit development. The results of this study suggest the opposite, a finding that now has been confirmed by other studies (e.g. Hossain and Nonami 2010).
Measuring growth and plant water status: the portable NMR sensor
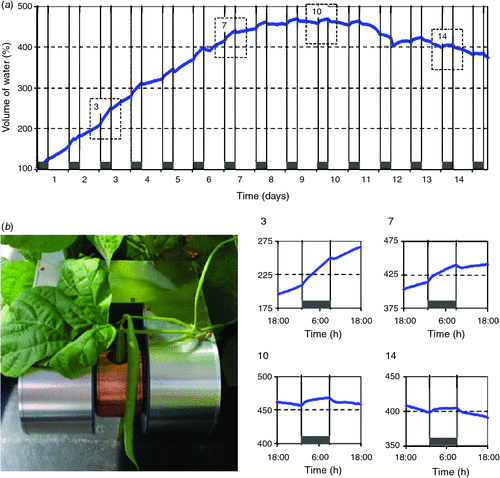
One of the most basic applications of magnetic resonance and one that is most easy to implement in a portable NMR device, is to use it as a non-spatially resolved water sensor (Windt et al. 2011). The amplitude of the NMR signal is linearly and quantitatively related to the total amount of liquid water in the scanner. In plants such a simple measurement can be surprisingly useful. It provides a unique way to measure growth non-invasively and to assess dynamic changes in the plant water status.
For proof-of-concept we present an experiment in which a 1-week-old bean pod was inserted into a portable NMR setup (Fig. 4b) and allowed to grow for 2 weeks during an automated measurement sequence (Fig. 4a). It should be noted that during the experiment the bean pod not only grew in thickness, but also in length. It rapidly grew from a length of 9.5 cm at t = 0 to 20 cm after 2 weeks, whereas the measurement only took place in the centre 15 mm. Thus, the graph does not represent the growth of the whole pod, but shows only the growth of a 15 mm section near the pod centre. Additionally, the rapid expansion of the pod caused it to move slightly during the experiment, giving a shift around day 12. For this reason, we here focus on timeframes in the growth curve, in which lengthening of the pod can be assumed to be negligible (Fig. 4a, detail graphs of day-night-day transitions 3, 7, 10 and 14). Towards the end of the second week, the amount of water in the pod was slowly decreasing. The amount of dry matter at that point, however, was still increasing (data not shown).
The day-night-day graphs exhibit several interesting features. Throughout the growth period the bean pod water content was affected strongly by light–dark cycle and during the night the growth rate always was larger than during the day. Towards the end of week 2, the pod even began losing water during the day and exhibited growth rates close to zero at night. The pod is known to transpire, but that alone is unlikely to cause such large changes in pod water content. Therefore, we conclude that throughout the whole growth period the pod remained apoplastically connected to the rest of the plant, thus, instantly experiencing the same diel changes in water potential as the vegetative part of the plant.
Quantifying transport processes with radiotracers
As described above, NMR techniques are suitable for studying water transport and giving structural information. Radiotracer technologies on the other hand help to better understand and quantify processes involved in long-distance transport and binding of specific compounds (e.g. recent photoassimilates). In particular, positron emitting radionuclides such as 11C (t1/2 = 20 min) or 13N (t1/2 = 10 min) are used for labelling C- or N-containing compounds, so that their spatial and temporal distribution in plants can be followed. The radioisotope 11C is especially suitable for plant studies since it provides traceable photoassimilates when administered to a leaf as 11CO2 in the light. Owing to the short half-life, 11C-experiments can be repeatedly performed on individual plants to show both diurnal and longer-term changes, for example in the distribution of photoassimilates as a plant develops (e.g. pea, Jahnke et al. 1989; wheat, Roeb and Britz 1991). By combining MRI with PET technology, it is possible to reveal both structural traits and transport functionality of bulky organs such as sugar beets and roots hidden in soil (Jahnke et al. 2009). Phloem and xylem transport of other short-lived radionuclides can be measured as well, such as 13N (Kiyomiya et al. 2001; Ohtake et al. 2001; Gómez et al. 2010), 15O (Nakanishi et al. 2002; Ohya et al. 2008) or 52Fe (Tsukamoto et al. 2009). Quantitative information and physiological parameters about metabolite fluxes, however, require robust analysis that allows for both the tracer’s short half-life and also its lack of equilibrium (e.g. Bühler et al. 2011).
Optimised usage of below-ground resources
On a global scale 50% of living plant material is found below ground where plant roots compete for spatially and temporally distributed resources (Walter et al. 2009). Plant roots obtain heterogeneously distributed resources via directed root growth, local promotion of rhizospheric metabolism to liberate resources, adjustment of uptake mechanisms, formation of mycorrhizal associations and by sensing neighbours and distributing their roots accordingly. Root phenotyping clearly has great potential in advancing our knowledge to clarify functional root responses to the environment. For example, a root system with an increased root growth, branching rate and root hair production is one option to optimise the uptake of water and nutrients. This may lead to an increased yield production provided that an acceptable balance in resource allocation between root and shoot is ensured (Lynch and Jonathan 2007). Additionally, water and nutrient capture and therefore yield can also be strongly affected not only by enhanced root growth but also by modifications in root angles (Hammer et al. 2009). Due to their hidden nature, root systems are less explored than the above-ground plant parts and the relevance of roots for enhancing yield seem to be underestimated as recent simulations show (Herder et al. 2010).
Quantifying the spatio-temporal dynamics of root growth and automated screening for optimised root system architecture
Approaches for studying the dynamics of root penetration into soil with a high spatial and temporal resolution are essential to achieve the goal of optimised resource use efficiency. Root growth takes place only at the root tip within a zone of a few millimetres in length. The permanent production and stream of new cells through the growth zone leads to a spatial distribution of cell expansion growth along the root tip (Silk 1992; Walter and Schurr 2005). The cellular expansion rate and, therefore, the temporal and spatial distribution of root growth are strongly affected by changes of environmental conditions, like temperature, water and nutrient availability, but also by light intensity or CO2 concentration at the shoot level (e.g. Muller et al. 1998; Sharp et al. 1988; Walter et al. 2002; Nagel et al. 2009; Walter et al. 2009). In order to quantify root growth adaptations we have developed a method with a high spatial (µm) and temporal (minutes) resolution based on digital image sequence processing (GROW MAP; Schmundt et al. 1998). The cell expansion growth is calculated from sequences of grey value images of root tips captured by digital cameras. Plants can be grown in conditions in which root tips are visible and accessible for the cameras, like in transparent agarose gels (Nagel et al. 2006), hydroponics (Walter et al. 2002), growth pouches (Hund et al. 2009) or in transparent rhizoboxes (Watt et al. 2006). In the acquired image sequences, cellular growth results in changes of local grey value structures. The first step of the analysis tool is the calculation of displacement vector fields for each image by optical flow. The resulting velocity vector fields are interpolated to fill in missing information and local growth rates are calculated by taking the divergence of the velocities of neighbouring pixels (Schmundt et al. 1998; Scharr 2007). This method has been successfully used to analyse root tip growth of several species, such as tobacco and maize (Walter et al. 2002; Nagel et al. 2006) and can easily be adapted to bean roots. The approach is suitable to elucidate how growth and development are affected by environmental factors and to find key genes (e.g. by forward genetics) that are for example responsible for abiotic stress tolerance of bean plants.
Plant root structure and function are tightly linked to abiotic stress tolerance, water and nutrient use efficiency and yield (Walter et al. 2009). Therefore, high-throughput phenotyping to screen large numbers of plant varieties and lines for bean genotypes with more beneficial root system architectures will be a valuable tool for breeding programs. Recently, imaging technologies have been developed that allow quantifying important features of root systems automatically (e.g. Armengaud et al. 2009; Nagel et al. 2009; Le Bot et al. 2010). The root system architecture of agar-grown plants can for example be analysed with the novel image-based software, GROWSCREEN-Root (Mühlich et al. 2008; Nagel et al. 2009). The key element of the software is the extraction of a tree model for root systems. Image sequences of entire root systems are acquired with a high resolution CCD camera and the origin of main roots is detected automatically as a local root element which is defined as a straight line of a few pixels’ length. Tracking the main root down to the root tip is automated by concatenating local root elements. Lateral roots branching from main roots axis are searched within a user-defined distance from the main root and tracked downwards to its tip in the same way as for the main root (for details see Mühlich et al. 2008; Nagel et al. 2009). This image-based software can quantify automatically the root system architecture by measuring root length and density, the distribution of roots within the substrate and branching rates (Fig. 5). In our example, we observed that 10 days after germination bean roots reached a total root length of ~1.5 m, a remarkable root growth potential highlighting the importance of detailed root growth studies even if limited to 2-D imaging methodologies. Such novel approaches when scaled to a desired throughput, will prove valuable in dedicated screening of bean root systems with optimised resource use efficiency.

|
Mapping the three-dimensional distribution of roots using MRI
In the sections above root growth was experimentally restricted to two dimensions allowing for straightforward investigation of basic root development. The spatial configuration of the developing root system, the root architecture, however, is of critical importance for plant performance, for example in soil resource acquisition by determining the extent of resource foraging in distinct soil domains (Lynch and Jonathan 2007). Currently, we can pre-select genotypes (based on their root-structure in specific environments) from large populations using high-throughput methods such as rhizotrons but for further and more detailed studies on root development and architecture imaging of roots in natural soil is required.
Root structures often lack symmetries and are dispersed in the opaque soil (Gregory 2006) posing considerable methodological problems. Modern 3-D imaging techniques such as magnetic resonance imaging (MRI) and computerised X-ray tomography (CT) offer solutions for basic research and phenotyping alike. Well known for its biomedical applications, MRI has occasionally also been used in plant sciences for studies ranging from imaging of fruits and shoots for quality and anatomical structure (Kuchenbrod et al. 1995; Kockenberger et al. 2004; Van As et al. 2009) to functional measurements of water status and flow in plant organs (Köckenberger 2001; Windt et al. 2006; Van As 2007). Several excellent textbooks describe the general principles of MRI (e.g. Haacke et al. 1999). Briefly, MRI is a volumetric imaging technique that enables detailed 3-D imaging of proton containing substances like water, but for which many substrates, including several soil types, are transparent. Roots in water containing soil can also be imaged since, due to different spatial positions of water relative to the soil particles which modulate the MRI-signal, different bodies of water (i.e. water in the soil and in plant roots) thus can be separated in MRI data.
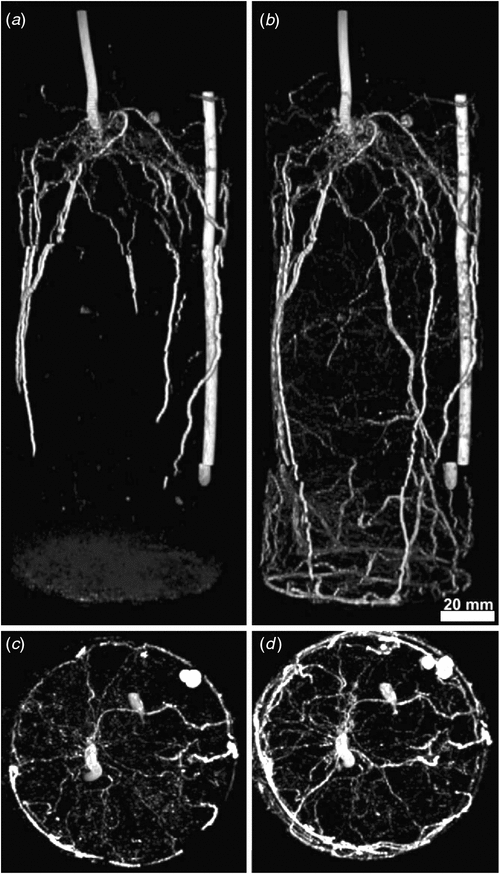
Few studies have investigated plant roots in (large) soil volumes, most likely because of the problem of local distortions of the magnetic field due to the presence of ferro-magnetic particles. Nevertheless, early in MRI-history, Bottomley et al. (1986) demonstrated the possibility of imaging roots of seedlings and young plants of Vicia faba L. potted in various substrates. With modern instruments and data analysis tools, we have now set up a dedicated laboratory for MRI on plants with a focus on root research and phenotyping. The customised Varian MRI-system (Palo Alto, CA, USA) used in our example on the root system of common bean plants (Phaseolus vulgaris L. cv. Fardenlosa shiny; Fig. 6) features a vertical bore 4.7 Tesla (T) superconducting magnet that can accommodate plants/pots up to a maximum of 17 cm diameter and 2 m length. Figure 3 shows 3-D images of a whole-root system and the lowest part of the hypocotyl of the same individual at an age of 14 (Fig. 6a) and 21 days (Fig. 6b). Image quality allows for a detailed characterisation of the root architecture, such as separating tap roots and basal roots and their individual distribution (Fig. 6). We estimate that currently roots down to a diameter of 300 µm could be imaged. After 21 days (Fig. 6b) roots tend to pack in the lower part of the cylinder, indicating that the container size may already be limiting for a relatively young plant. Projection of root distribution in the axial plane (Figs 6c, d) provides a simpler visualisation of the angular distribution of roots and their development.
|
Imaging the whole-root system allows extraction of different parameters from the same measurement session: number and length of different root types, branching angles, root mass and their spatial distribution. The current spatial resolution would be sufficient for studying development and distribution of root-nodules in legumes hosting N2-fixing root symbionts (the plant shown here was not nodulated). The non-invasive nature of MRI allows the study of individual root (system) development and thereby also dynamic responses to stress and environmental conditions (including the vicinity of other plants, soil fauna and abiotic stress). This will enable us, for the first time, to follow the complex 3-D development of below-ground structures in vivo for gaining a mechanistic understanding of interactions between the root system and its abiotic and biotic environment.
In addition to root–soil interactions, pronounced plant-plant interactions between species can occur (e.g. de Kroon 2007; Bezemer et al. 2010). Results from grassland biodiversity experiments have shown positive effects of plant diversity on overall productivity and nutrient cycling (Roscher et al. 2011), mainly related to complementary use of overall resources between species with different resource foraging traits and to beneficial effects of N2-fixing legume presence. We used MRI to map the development of root systems over time of two different plant individuals (either of the same species or of different species) compared with one individual (control). We addressed the hypothesis that growth with an interspecific neighbour will result in increased root growth away from that neighbour’s roots (avoidance), vs a lesser effect with a conspecific neighbour. 3-D images allowed individual-specific observation of tap and lateral root development over time (Fig. 7). For a quantifiable inter-treatment comparison, the 3-D data cubes were translated into 2-D images and developed an algorithm for separating the diameter of the tube into three zones (Fig. 7c, f) for calculating the percentage of total root mass found in each zone. This now allows a comparison of the effects of interacting species identity on overall root distribution.
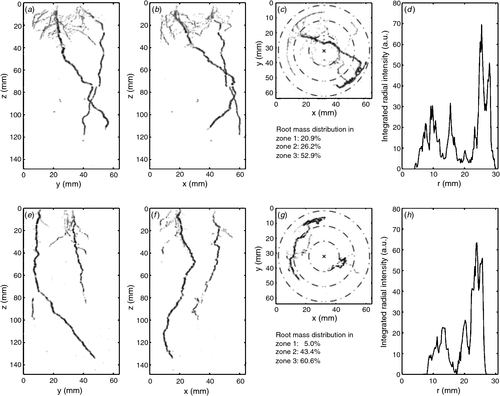
|
Thus, we believe that root MRI, especially when used with automated image analysis tools, will enhance the mechanistic understanding of development and architecture of the root system and its interaction with the soil and neighbouring plants. These methods provide us with tools for non-invasive, high-precision imaging of dynamics of the ‘hidden half’. Insights from MRI studies will be of high ecological and economical value because resources such as water and mineral nutrients are becoming increasingly limited in many soils and expensive in agriculture (Lynch and Jonathan 2007) and an understanding of the response of root development to biotic and abiotic factors can indicate how and where fossil fuel-based fertilisers could be complemented by positive plant–plant interactions and by ‘green fertilisers’ (N2-fixing legumes) to supplement and sustain crop species development.
The challenge of measuring root distribution in the field
Mapping root structure in the field remains an unsolved challenge. Root growth and root function in natural soils are known to be fundamentally different to root growth in pots, which stresses the importance of developing approaches to quantify root distribution and function in nature. To our knowledge, there is no method available at the moment that allows a non-invasive mapping of root systems in natural soils. Researchers still have to dig out soil cores and manually score root traits (Trachsel et al. 2011), which does not allow monitoring of root growth and development and does not address the 3-D structure. Additionally, fine roots that constitute the functionally most important component of the root system are destroyed by destructive measurements and there is no method available to directly assess the fine root system (Hendricks et al. 1993). One potentially useful approach may emerge from NMR technologies. For example, the oil industry spent substantial effort in the past decades to adapt so called inside-out NMR techniques for the exploration of oil wells (Brown et al. 2001). This technology was recently transferred to map water distribution and pore size in natural soils (Haber et al. 2010). Even though still far from a routine application, this approach may permit mapping the 3-D structure of roots in the field.
Conclusions
Maximising yield per unit of resource use will remain a main focus of future plant improvement programs. Phenotyping methodologies that can feasibly be scaled up to achieve higher throughput (such as several of those presented here) will form an essential component of such programs, by integrating spatial and temporal patterns in plant development and function that are subject to dynamic resource limitations. Despite significant challenges to the development of such in situ techniques, interdisciplinary approaches provide great promise for the development of practical, usable tools to monitor above-ground, below-ground and transport processes.
Several non-invasive phenotyping sensors and protocols can be adapted to bean populations and breeding material. Quantifying root growth in relation to resource availability may help to understand how beans exploit below-ground nutrients and interact with their neighbours. Imaging spectroscopy will further our understanding of how leaves and shoots are affected by environmental constraints and of spatio-temporal stress responses of plants and canopies. Non-invasive and spatially resolved measurements of transport processes and resource allocation may be used to better understand and potentially guide transport between plant organs and pods. Finally, we stress that single high-throughput phenotyping pipelines (e.g. high-throughput estimation of shoot biomass) are useful, but that only the combination of several phenotyping protocol ‘chains’ and at different scales (from semi-controlled environments to field) will significantly contribute to a deeper understanding of the dynamic processes in both individual plants and canopies.
Acknowledgements
This work has been made possible by the funding support of the BMBF Network CropSense and the DAAD fellowship to Francisco Pinto. Measurements of Fig. 2 (Soy-FACE) were supported by the Illinois Council for Food and Agricultural Research, the U.S. Department of Agricultural, and the Illinois Agricultural Experiment Station. The authors also greatly thank Bernd Kastenholz for cultivation of bean plants on agar for root system analysis; Jonas Bühler for developing the quantification algorithm of the data shown in Fig. 7 and Lena Meck for editing the manuscript.
References
Ainsworth EA, Yendrek CR, Skoneczka JA, Long SP (2011) Accelerating yield potential in soybean: potential targets for biotechnological improvement. Plant, Cell & Environment| Accelerating yield potential in soybean: potential targets for biotechnological improvement.Crossref | GoogleScholarGoogle Scholar |
Alonso L, Gomez-Chova L, Vila-Frances J, Amoros-Lopez J, Guanter L, Calpe J, Moreno J (2008) Improved Fraunhofer line discrimination method for vegetation fluorescence quantification. IEEE Geoscience and Remote Sensing Letters 5, 620–624.
| Improved Fraunhofer line discrimination method for vegetation fluorescence quantification.Crossref | GoogleScholarGoogle Scholar |
Ananyev G, Kolber ZS, Klimov D, Falkowski PG, Berry JA, Rascher U, Martin R, Osmond CB (2005) Remote sensing of heterogeneity in photosynthetic efficiency, electron transport and dissipation of excess light in Populus deltoides stands under ambient and elevated CO2 concentrations, and in a tropical forest canopy, using a new laser-induced fluorescence transient device. Global Change Biology 11, 1195–1206.
| Remote sensing of heterogeneity in photosynthetic efficiency, electron transport and dissipation of excess light in Populus deltoides stands under ambient and elevated CO2 concentrations, and in a tropical forest canopy, using a new laser-induced fluorescence transient device.Crossref | GoogleScholarGoogle Scholar |
Armengaud P, Zambaux K, Hills A, Sulpice R, Pattison RJ, Blatt MR, Amtmann A (2009) EZ-Rhizo: integrated software for the fast and accurate measurement of root system architecture. The Plant Journal 57, 945–956.
| EZ-Rhizo: integrated software for the fast and accurate measurement of root system architecture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjslSltr0%3D&md5=8793380f6a2fdd3c8ac709de57e00c2cCAS |
Asner GP, Vitousek PM (2005) Remote analysis of biological invasion and biogeochemical change. Proceedings of the National Academy of Sciences of the United States of America 102, 4383–4386.
| Remote analysis of biological invasion and biogeochemical change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXivFCrtbs%3D&md5=b97b395f9ee5b241c0025f01a4f82a6bCAS |
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology 59, 89–113.
| Chlorophyll fluorescence: a probe of photosynthesis in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFaqsL8%3D&md5=cb401be9e3802e2a6dd7d60ad3a2e810CAS |
Bezemer TM, Fountain MT, Barea JM, Christensen S, Dekker SC, Duyts H, van Hal R, Harvey JA, Hedlund K, Maraun M, Mikola J, Mladenov AG, Robin C, de Ruiter PC, Scheu S, Setälä H, Šmilauer P, van der Putten WH (2010) Divergent composition but similar function of soil food webs beneath individual plants: plant species and community effects. Ecology 91, 3027–3036.
| Divergent composition but similar function of soil food webs beneath individual plants: plant species and community effects.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cblslSmtQ%3D%3D&md5=ae2cb5c68489a6c61c9b6ee1e9605a66CAS |
Biskup B, Scharr H, Schurr U, Rascher U (2007) A stereo imaging system for measuring structural parameters of plant canopies. Plant, Cell & Environment 30, 1299–1308.
| A stereo imaging system for measuring structural parameters of plant canopies.Crossref | GoogleScholarGoogle Scholar |
Biskup B, Scharr H, Fischbach A, Wiese-Klinkenberg A, Schurr U, Walter A (2009) Diel growth cycle of isolated leaf discs analyzed with a novel, high-throughput three-dimensional imaging method is identical to that of intact leaves. Plant Physiology 149, 1452–1461.
| Diel growth cycle of isolated leaf discs analyzed with a novel, high-throughput three-dimensional imaging method is identical to that of intact leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsFCiur0%3D&md5=5388dd2d6485a45351e50f9a2a62816cCAS |
Bottomley PA, Rogers HH, Foster TH (1986) NMR imaging shows water distribution and transport in plant roof systems in situ. Proceedings of the National Academy of Sciences of the United States of America 83, 87–89.
| NMR imaging shows water distribution and transport in plant roof systems in situ.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cnivFamuw%3D%3D&md5=7409e71df21b151a12b225189f5ac289CAS |
Bouguet JY (2005) Camera calibration toolbox for Matlab. Available at http://www.vision.caltech.edu/bouguetj/calib_doc/ [Verified 28 October 2011]
Brown RJS, Chandler R, Jackson JA, Kleinberg RL, Miller MN, Paltiel Z, Prammer MG (2001) The history of NMR well logging. Concepts of Magnetic Resoncance 13, 335–413.
Brown MZ, Burschka D, Hager GD (2003) Advances in computational stereo. IEEE Transactions on Pattern Analysis and Machine Intelligence 25, 993–1008.
| Advances in computational stereo.Crossref | GoogleScholarGoogle Scholar |
Bühler J, Huber G, Schmid F, Blümler P (2011) Analytical model for long-distance tracer-transport in plants. Journal of Theoretical Biology 270, 70–79.
| Analytical model for long-distance tracer-transport in plants.Crossref | GoogleScholarGoogle Scholar |
Curran PJ (1989) Remote-sensing of foliar chemistry. Remote Sensing of Environment 30, 271–278.
| Remote-sensing of foliar chemistry.Crossref | GoogleScholarGoogle Scholar |
Damm A, Elbers J, Erler E, Gioli B, Hamdi K, Hutjes R, Kosvancova M, Meroni M, Miglietta F, Moersch A, Moreno J, Schickling A, Sonnenschein R, Udelhoven T, van der Linden S, Hostert P, Rascher U (2010) Remote sensing of sun induced fluorescence to improve modeling of diurnal courses of gross primary production (GPP). Global Change Biology 16, 171–186.
| Remote sensing of sun induced fluorescence to improve modeling of diurnal courses of gross primary production (GPP).Crossref | GoogleScholarGoogle Scholar |
de Kroon H (2007) How do roots interact? Science 318, 1562–1563.
| How do roots interact?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVemtLjL&md5=f6ae159fd380cfc363a003abbbf3cc21CAS |
Dermody O, Long SP, McConnaughay K, Delucia EH (2008) How do elevated CO2 and O3 affect the interception and utilization of radiation by a soybean canopy? Global Change Biology 14, 556–564.
| How do elevated CO2 and O3 affect the interception and utilization of radiation by a soybean canopy?Crossref | GoogleScholarGoogle Scholar |
Duvick DN (2005) The contribution of breeding to yield advances in maize (Zea mays L.). Advances in Agronomy 86, 83–145.
| The contribution of breeding to yield advances in maize (Zea mays L.).Crossref | GoogleScholarGoogle Scholar |
Ehleringer JR, Forseth IN (1980) Solar tracking by plants. Science 210, 1094–1098.
| Solar tracking by plants.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvlsVegtg%3D%3D&md5=573f01e3407fe9447cb37b36215a2e93CAS |
Evans LT (1997) Adapting and improving crops: the endless task. Philosophical Transactions of the Royal Society B. Biological Sciences 352, 901–906.
| Adapting and improving crops: the endless task.Crossref | GoogleScholarGoogle Scholar |
Felzenszwalb PF, Huttenlocher D (1998) Image segmentation using local variation. In ‘Proceedings of IEEE Conference on Computer Vision and Pattern Recognition’. pp. 98–104. (IEEE Computer Society: Washington, DC)
Flexas J, Briantais JM, Cerovic Z, Medrano H, Moya I (2000) Steady-state and maximum chlorophyll fluorescence responses to water stress in grapevine leaves: a new remote sensing system. Remote Sensing of Environment 73, 283–297.
| Steady-state and maximum chlorophyll fluorescence responses to water stress in grapevine leaves: a new remote sensing system.Crossref | GoogleScholarGoogle Scholar |
Frak E, Le Roux X, Millard P, Adam B, Dreyer E, Escuit C, Sinoquet H, Vandame M, Varlet-Grancher C (2002) Spatial distribution of leaf nitrogen and photosynthetic capacity within the foliage of individual trees: disentangling the effects of local light quality, leaf irradiance, and transpiration. Journal of Experimental Botany 53, 2207–2216.
| Spatial distribution of leaf nitrogen and photosynthetic capacity within the foliage of individual trees: disentangling the effects of local light quality, leaf irradiance, and transpiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xpt1Kgtb4%3D&md5=3ec148e91b1f9c8911473dcac5be1f3dCAS |
Franke J, Menz G, Oerke EC, Rascher U (2005) Comparison of multi- and hyperspectral imaging data of leaf rust infected wheat plants. In ‘Remote Sensing for Agriculture, Ecosystems, and Hydrology VII. Proceedings of SPIE Vol. 5976’. (Eds M Owe, G D’Urso) pp. 341–350. (SPIE Press: Brugge, Belgium)
Fua P (1993) A parallel stereo algorithm that produces dense depth maps and preserves image features. Machine Vision and Applications 6, 35–49.
| A parallel stereo algorithm that produces dense depth maps and preserves image features.Crossref | GoogleScholarGoogle Scholar |
Gamon JA, Penuelas J, Field CB (1992) A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sensing of Environment 41, 35–44.
| A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency.Crossref | GoogleScholarGoogle Scholar |
Gilbert ME, Zwieniecki MA, Holbrook NM (2011) Independent variation in photosynthetic capacity and stomatal conductance leads to differences in intrinsic water use efficiency in 11 soybean genotypes before and during mild drought. Journal of Experimental Botany 62, 2875–2887.
| Independent variation in photosynthetic capacity and stomatal conductance leads to differences in intrinsic water use efficiency in 11 soybean genotypes before and during mild drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmsVyksbY%3D&md5=3d27b99c5f6c3e39017af035f98dae28CAS |
Gitelson AA, Merzlyak MN, Chivkunova OB (2001) Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochemistry and Photobiology 74, 38–45.
| Optical properties and nondestructive estimation of anthocyanin content in plant leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltVyks7c%3D&md5=23c4a25e3de9e702b8744b2b6f21d030CAS |
Gitelson AA, Zur Y, Chivkunova OB, Merzlyak MN (2002) Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochemistry and Photobiology 75, 272–281.
| Assessing carotenoid content in plant leaves with reflectance spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivFGntLk%3D&md5=03a72025db36c5359a5369d702ea8cb7CAS |
Godfray HC, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327, 812–818.
| Food security: the challenge of feeding 9 billion people.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhslWisLo%3D&md5=d9ff1a2cec0b1ee06fd564d71097dde5CAS |
Gómez S, Ferrieri RA, Schueller M, Orians CM (2010) Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato. New Phytologist 188, 835–844.
| Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato.Crossref | GoogleScholarGoogle Scholar |
Good AG, Shrawat AK, Muench DG (2004) Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends in Plant Science 9, 597–605.
| Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVaksLvM&md5=6011cfb7ae08b7ebb9b39f1cf245a5c6CAS |
Gowik U, Westhoff P (2011) The path from C3 to C4 photosynthesis. Plant Physiology 155, 56–63.
| The path from C3 to C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFagsL0%3D&md5=3543a8ad4e52a510777dcd50b4241b48CAS |
Gregory PJ (2006) ‘Plant roots.’ (Blackwell Publishing Ltd: Oxford)
Haacke EM, Brown RW, Thompson MR, Venkatesan R (1999) ‘Magnetic resonance imaging.’ (John Wiley & Sons: New York)
Haber A, Haber-Pohlmeier S, Casanova F, Blumich B (2010) Relaxation-Relaxation Experiments in Natural Porous Media with Portable Halbach Magnets. Vadose Zone Journal 9, 893–897.
| Relaxation-Relaxation Experiments in Natural Porous Media with Portable Halbach Magnets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltVajtb0%3D&md5=b2e6c0e3ef07e775be4dc845baf4bf3bCAS |
Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt? Crop Science 49, 299–312.
| Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt?Crossref | GoogleScholarGoogle Scholar |
Hartley RI, Zisserman A (2004) ‘Multiple view geometry in computer vision.’ (Cambridge University Press: Cambridge, UK)
Hendricks JJ, Nadelhoffer KJ, Aber JD (1993) Assessing the role of fine roots in carbon and nutrient cycling. Trends in Ecology & Evolution 8, 174–178.
| Assessing the role of fine roots in carbon and nutrient cycling.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itVylug%3D%3D&md5=e8d829fc6a4250fcf9839582bada4396CAS |
Herder GD, Isterdael GV, Beeckman T, De Smet I (2010) The roots of a new green revolution. Trends in Plant Science 15, 600–607.
| The roots of a new green revolution.Crossref | GoogleScholarGoogle Scholar |
Hibberd JM, Sheehy JE, Langdale JA (2008) Using C4 photosynthesis to increase the yield of rice – rationale and feasibility. Current Opinion in Plant Biology 11, 228–231.
| Using C4 photosynthesis to increase the yield of rice – rationale and feasibility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktFCmsLs%3D&md5=c63febccc6ae6adabd60552017733147CAS |
Hirel B, Le Gouis J, Ney B, Gallais A (2007) The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. Journal of Experimental Botany 58, 2369–2387.
| The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXos1ykt74%3D&md5=060a0fbca0ad97e51e940521324f1e24CAS |
Hossain MM, Nonami H (2010) Effects of water flow from the xylem on the growth-induced water potential and the growth-effective turgor associated with enlarging tomato fruit. Environment Control in Biology 48, 101–116.
| Effects of water flow from the xylem on the growth-induced water potential and the growth-effective turgor associated with enlarging tomato fruit.Crossref | GoogleScholarGoogle Scholar |
Houle D, Govindaraju DR, Omholt S (2010) Phenomics: the next challenge. Nature Reviews. Genetics 11, 855–866.
| Phenomics: the next challenge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVejs7jK&md5=08fb3c5b88385bf5df372ea31770143dCAS |
Hund A, Trachsel S, Stamp P (2009) Growth of axile and lateral roots of maize: I. Development of a phenotying platform. Plant and Soil 325, 335–349.
| Growth of axile and lateral roots of maize: I. Development of a phenotying platform.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFSrt7vI&md5=09fa0807116a281710bd93a4cc731b5eCAS |
Jahnke S, Bier D, Estruch JJ, Beltran JP (1989) Distribution of photoassimilates in the pea plant: chronology of events in non-fertilized ovaries and effects of gibberellic acid. Planta 180, 53–60.
| Distribution of photoassimilates in the pea plant: chronology of events in non-fertilized ovaries and effects of gibberellic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlt1elsA%3D%3D&md5=a0995707d05df790e909c93704d72243CAS |
Jahnke S, Menzel MI, van Dusschoten D, Roeb GW, Bühler J, Minwuyelet S, Blümler P, Temperton VM, Hombach T, Streun M, Beer S, Khodaverdi M, Ziemons K, Coenen HH, Schurr U (2009) Combined MRI-PET dissects dynamic changes in plant structures and functions. The Plant Journal 59, 634–644.
| Combined MRI-PET dissects dynamic changes in plant structures and functions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFChtrrK&md5=10c7273c93e4a049e922b9f3131efbfdCAS |
Jansen M, Gilmer F, Biskup B, Nagel KA, Rascher U, Fischbach A, Briem S, Dreissen G, Tittmann S, Braun S, De Jaeger I, Metzlaff M, Schurr U, Scharr H, Walter A (2009) Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants. Functional Plant Biology 36, 902–914.
| Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlOgs7rF&md5=85fe567810d5e3477205ea5a4434545aCAS |
Kant S, Bi YM, Rothstein SJ (2011) Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. Journal of Experimental Botany 62, 1499–1509.
| Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1Gjsbs%3D&md5=5a372059222797783e680f13014958d2CAS |
Kao WY, Forseth IN (1991) The effect of nitrogen, light and water availability on tropic leaf movement in soybean (Glycine max). Plant, Cell & Environment 14, 287–293.
| The effect of nitrogen, light and water availability on tropic leaf movement in soybean (Glycine max).Crossref | GoogleScholarGoogle Scholar |
Kao WY, Forseth IN (1992) Diurnal leaf movement, chlorophyll fluorescence and carbon assimilation in soybean grown under different nitrogen and water availabilities. Plant, Cell & Environment 15, 703–710.
| Diurnal leaf movement, chlorophyll fluorescence and carbon assimilation in soybean grown under different nitrogen and water availabilities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xls1Oqtr4%3D&md5=0565731b8a928cf30e74f62b4cfa58e9CAS |
Kiyomiya S, Nakanishi H, Uchida H, Tsuji A, Nishiyama S, Futatsubashi M, Tsukada H, Ishioka NS, Watanabe S, Ito T, Mizuniwa C, Osa A, Matsuhashi S, Hashimoto S, Sekine T, Mori S (2001) Real time visualization of 13N-translocation in rice under different environmental conditions using positron emitting tracer imaging system. Plant Physiology 125, 1743–1753.
| Real time visualization of 13N-translocation in rice under different environmental conditions using positron emitting tracer imaging system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtFKqtL8%3D&md5=c2897975c5d01aea23f6f8d0dbbd9885CAS |
Knoblauch M, Peters WS, Ehlers K, van Bel AJE (2001) Reversible calcium-regulated stopcocks in legume sieve tubes. The Plant Cell 13, 1221–1230.
Köckenberger W (2001) Functional imaging of plants by magnetic resonance experiments. Trends in Plant Science 6, 286–292.
| Functional imaging of plants by magnetic resonance experiments.Crossref | GoogleScholarGoogle Scholar |
Kockenberger W, De Panfilis C, Santoro D, Dahiya P, Rawsthorne S (2004) High resolution NMR microscopy of plants and fungi. Journal of Microscopy 214, 182–189.
| High resolution NMR microscopy of plants and fungi.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c3gsFajug%3D%3D&md5=85a783700ed11d7d51afc8b568634f4cCAS |
Kolber Z, Klimov D, Ananyev G, Rascher U, Berry JA, Osmond CB (2005) Measuring photosynthetic parameters at a distance: laser induced fluorescence transient (LIFT) method for remote measurements of PSII in terrestrial vegetation. Photosynthesis Research 84, 121–129.
| Measuring photosynthetic parameters at a distance: laser induced fluorescence transient (LIFT) method for remote measurements of PSII in terrestrial vegetation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXms1yitrs%3D&md5=f5b54aca1d7ebe3462619c8addb397b4CAS |
Körner C (2011) The grand challenges in functional plant ecology. Frontiers in Plant Sciences 2, 1–3.
| The grand challenges in functional plant ecology.Crossref | GoogleScholarGoogle Scholar |
Kuchenbrod E, Haase A, Benkert R, Schneider H, Zimmermann U (1995) Quantitative NMR microscopy on intact plants. Magnetic Resonance Imaging 13, 447–455.
| Quantitative NMR microscopy on intact plants.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MzhtFKktw%3D%3D&md5=0da89bbf69fad484ec489b4eb6abf0f7CAS |
Le Bot J, Serra V, Fabre J, Draye X, Adamowicz S, Pagès L (2010) DART: a software to analyse root system architecture and development from captured images. Plant and Soil 326, 261–273.
| DART: a software to analyse root system architecture and development from captured images.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGrt7jE&md5=55752bea5f6d84bf4178ad281780333fCAS |
Liu LY, Zhang YJ, Wang JH, Zhao CJ (2005) Detecting solar-induced chlorophyll fluorescence from field radiance spectra based on the Fraunhofer line principle. IEEE Transactions on Geoscience and Remote Sensing 43, 827–832.
| Detecting solar-induced chlorophyll fluorescence from field radiance spectra based on the Fraunhofer line principle.Crossref | GoogleScholarGoogle Scholar |
Long SP, Ort DR (2010) More than taking the heat: crops and global change. Current Opinion in Plant Biology 13, 240–247.
| More than taking the heat: crops and global change.Crossref | GoogleScholarGoogle Scholar |
Long SP, Zhu X-G, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant, Cell & Environment 29, 315–330.
| Can improvement in photosynthesis increase crop yields?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xktlyltrw%3D&md5=9f5c649085a34b282eede80180c02b0cCAS |
Lynch JP, Jonathan P (2007) Roots of the second green revolution. Australian Journal of Botany 55, 493–512.
| Roots of the second green revolution.Crossref | GoogleScholarGoogle Scholar |
Maier SW, Günther KP, Stellmes M (2003) Sun-induced fluorescence: a new tool for precision farming. In ‘Digital imaging and spectral techniques: applications to precision agriculture and crop physiology’. (Eds T van Toai, D Major, M McDonald, J Schepers, L Tarpley) pp. 209–222. (American Society of Agronomy: Madison, WI)
Malenovsky Z, Mishra KB, Zemek F, Rascher U, Nedbal L (2009) Scientific and technical challenges in remote sensing of plant canopy reflectance and fluorescence. Journal of Experimental Botany 60, 2987–3004.
| Scientific and technical challenges in remote sensing of plant canopy reflectance and fluorescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsValsbg%3D&md5=2cd4409b92442cfcc47a6916863efcb4CAS |
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Annals of Botany 105, 1141–1157.
| Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture.Crossref | GoogleScholarGoogle Scholar |
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany 51, 659–668.
| Chlorophyll fluorescence – a practical guide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtF2js74%3D&md5=092bf5f89ae722b319a6295d4f24cbd4CAS |
Meroni M, Colombo R (2006) Leaf level detection of solar induced chlorophyll fluorescence by means of a subnanometer resolution spectroradiometer. Remote Sensing of Environment 103, 438–448.
| Leaf level detection of solar induced chlorophyll fluorescence by means of a subnanometer resolution spectroradiometer.Crossref | GoogleScholarGoogle Scholar |
Meroni M, Rossini M, Guanter L, Alonso L, Rascher U, Colombo R, Moreno J (2009) Remote sensing of solar-induced chlorophyll fluorescence: review of methods and applications. Remote Sensing of Environment 113, 2037–2051.
| Remote sensing of solar-induced chlorophyll fluorescence: review of methods and applications.Crossref | GoogleScholarGoogle Scholar |
Mittler R, Blumwald E (2010) Genetic engineering for modern agriculture: challenges and perspectives. Annual Review of Plant Biology 61, 443–462.
| Genetic engineering for modern agriculture: challenges and perspectives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnslSjsLc%3D&md5=834ce88b3b10f8445d8b288a4f51146fCAS |
Moll RH, Kamprath EJ, Jackson WA (1982) Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agronomy Journal 74, 562–564.
| Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization.Crossref | GoogleScholarGoogle Scholar |
Moya I, Camenen L, Evain S, Goulas Y, Cerovic ZG, Latouche G, Flexas J, Ounis A (2004) A new instrument for passive remote sensing 1. Measurements of sunlight-induced chlorophyll fluorescence. Remote Sensing of Environment 91, 186–197.
| A new instrument for passive remote sensing 1. Measurements of sunlight-induced chlorophyll fluorescence.Crossref | GoogleScholarGoogle Scholar |
Mühlich M, Truhn D, Nagel KA, Walter A, Scharr H, Aach T (2008) Measuring plant root growth. In ‘Lecture notes in Computer Science 5096’. (Ed. G Rigoll) pp. 497–506. (Springer: Heidelberg, Germany)
Muller B, Stosser M, Tardieu F (1998) Spatial distributions of tissue expansion and cell division rates are related to irradiance and to sugar content in the growing zone of maize roots. Plant, Cell & Environment 21, 149–158.
| Spatial distributions of tissue expansion and cell division rates are related to irradiance and to sugar content in the growing zone of maize roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjslGgtLc%3D&md5=d85de6f708962687a3cfdf0c4875aad2CAS |
Murchie EH, Pinto M, Horton P (2009) Agriculture and the new challenges for photosynthesis research. New Phytologist 181, 532–552.
| Agriculture and the new challenges for photosynthesis research.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivFSls70%3D&md5=14d29d2e1a4f69f82e79fa3894a1824dCAS |
Nagel KA, Schurr U, Walter A (2006) Dynamics of root growth stimulation in Nicotiana tabacum in increasing light intensity. Plant, Cell & Environment 29, 1936–1945.
| Dynamics of root growth stimulation in Nicotiana tabacum in increasing light intensity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1aktLjJ&md5=dcae25c8c7e05ac78bffe78e9f613ad5CAS |
Nagel KA, Kastenholz B, Jahnke S, van Dusschoten D, Aach T, Mühlich M, Truhn D, Scharr H, Terjung S, Walter A, Schurr U (2009) Temperature responses of roots: impact on growth, root system architecture and implications for phenotyping. Functional Plant Biology 36, 947–959.
| Temperature responses of roots: impact on growth, root system architecture and implications for phenotyping.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlOgs7vM&md5=4a68526c6c350fa9f16c551c5cbbbb3cCAS |
Nakanishi H, Kiyomiya S, Tsukamoto T, Tsukada H, Uchida H, Mori S (2002) Water (H215O) flow in rice is regulated by the concentration of nutrients as monitored by positron multi-probe system (PMPS). Soil Science and Plant Nutrition 48, 759–762.
Ohtake N, Sato T, Fujikake H, Sueyoshi K, Ohyama T, Ishioka NS, Watanabe S, Osa A, Sekine T, Matsuhashi S, Ito T, Mizuniwa C, Kume T, Hashimoto S, Uchida H, Tsuji A (2001) Rapid N transport to pods and seeds in N-deficient soybean plants. Journal of Experimental Botany 52, 277–283.
| Rapid N transport to pods and seeds in N-deficient soybean plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtVajurg%3D&md5=9ac3d00b3053a931124b4d31f1f03ad1CAS |
Ohya T, Tanoi K, Hamada Y, Okabe H, Rai H, Hojo J, Suzuki K, Nakanishi TM (2008) An analysis of long-distance water transport in the soybean stem using H215O. Plant & Cell Physiology 49, 718–729.
| An analysis of long-distance water transport in the soybean stem using H215O.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvFWns70%3D&md5=dc21a18298518bc3fcf492f94fa0054dCAS |
Olson CF, Matthies LH, Wright JR, Li R, Di K (2007) Visual terrain mapping for mars exploration. Computer Vision and Image Understanding 105, 73–85.
| Visual terrain mapping for mars exploration.Crossref | GoogleScholarGoogle Scholar |
Osmond CB, Björkman O, Anderson DJ (1980) ‘Physiological processes in plant ecology.’ (Springer: New York)
Parry MAJ, Andralojc PJ, Mitchell RAC, Madgwick PJ, Keys AJ (2003) Manupulation of Rubisco: the amount, activity, function and regulation. Journal of Experimental Botany 54, 1321–1333.
| Manupulation of Rubisco: the amount, activity, function and regulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjt1Ogs7s%3D&md5=ddaf7758496fe6d20d68fb7de5327801CAS |
Pieruschka R, Klimov D, Kolber ZS, Berry JA (2010) Monitoring of cold and light stress impact on photosynthesis by using the laser induced fluorescence transient (LIFT) approach. Functional Plant Biology 37, 395–402.
| Monitoring of cold and light stress impact on photosynthesis by using the laser induced fluorescence transient (LIFT) approach.Crossref | GoogleScholarGoogle Scholar |
Plascyk JA, Gabriel FC (1975) The Fraunhofer line discriminator MKII – an airborne instrument for precise and standardized ecological luminescence measurement. IEEE Transactions on Instrumentation and Measurement 24, 306–313.
| The Fraunhofer line discriminator MKII – an airborne instrument for precise and standardized ecological luminescence measurement.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Niinemets Ü, Walter A, Fiorani F, Schurr U (2010) A method to construct dose–response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data. Journal of Experimental Botany 61, 2043–2055.
| A method to construct dose–response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVGgu7c%3D&md5=184d20864d5cb74cabca58d5d4d8887dCAS |
Rascher U, Nedbal L (2006) Dynamics of photosynthesis in fluctuating light – commentary. Current Opinion in Plant Biology 9, 671–678.
| Dynamics of photosynthesis in fluctuating light – commentary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVygt7jF&md5=2f74693d8c6aa0a395430c1094b1c055CAS |
Rascher U, Pieruschka R (2008) Spatio-temporal variations of photosynthesis – the potential of optical remote sensing to better understand and scale light use efficiency and stresses of plant ecosystems. Precision Agriculture 9, 355–366.
| Spatio-temporal variations of photosynthesis – the potential of optical remote sensing to better understand and scale light use efficiency and stresses of plant ecosystems.Crossref | GoogleScholarGoogle Scholar |
Rascher U, Nichol CJ, Small C, Hendricks L (2007) Monitoring spatio-temporal dynamics of photosynthesis with a portable hyperspectral imaging system. Photogrammetric Engineering and Remote Sensing 73, 45–56.
Rascher U, Agati G, Alonso L, Cecchi G, Champagne S, Colombo R, Damm A, Daumard F, de Miguel E, Fernandez G, Franch B, Franke J, Gerbig C, Gioli B, Gómez JA, Goulas Y, Guanter L, Gutiérrez-De-La-Cámara Ó, Hamdi K, Hostert P, Jiménez M, Kosvancova M, Lognoli D, Meroni M, Miglietta F, Moersch A, Moreno J, Moya I, Neininger B, Okujeni A, Ounis A, Palombi L, Raimondi V, Schickling A, Sobrino JA, Stellmes M, Toci G, Toscano P, Udelhoven T, van der Linden S, Zaldei A (2009) CEFLES2: the remote sensing component to quantify photosynthetic efficiency from the leaf to the region by measuring sun-induced fluorescence in the oxygen absorption bands. Biogeosciences 6, 1181–1198.
| CEFLES2: the remote sensing component to quantify photosynthetic efficiency from the leaf to the region by measuring sun-induced fluorescence in the oxygen absorption bands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1OhtLrL&md5=ce8488703056407719f2e38205c28e16CAS |
Rascher U, Biskup B, Leakey ADB, McGrath JM, Ainsworth EA (2010a) Altered physiological function, not structure, drives increased radiation-use efficiency of soybean grown at elevated CO2. Photosynthesis Research 105, 15–25.
| Altered physiological function, not structure, drives increased radiation-use efficiency of soybean grown at elevated CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntlGitLs%3D&md5=6e043e21e5c0de39086b80c8467ae618CAS |
Rascher U, Damm A, van der Linden S, Okujeni A, Pieruschka R, Schickling A, Hostert P (2010b) Sensing of photosynthetic activity of crops. In ‘Precision crop protection – the challenge and use of heterogeneity’. (Ed. EC Oerke) (Springer Science + Business Media BV: Dordrecht, The Netherlands)
Roeb G, Britz SJ (1991) Short-term fluctuations in the transport of assimilates to the ear of wheat measured with steady state 11C–CO2-labelling of the flag leaf. Journal of Experimental Botany 42, 469–475.
| Short-term fluctuations in the transport of assimilates to the ear of wheat measured with steady state 11C–CO2-labelling of the flag leaf.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXisVCmsrc%3D&md5=d938ec1213aabbe258469a8374cc6420CAS |
Rogers A, Allen DJ, Davey PA, Morgan PB, Ainsworth EA, Bernacchi CJ, Cornic G, Dermody O, Dohleman FG, Heaton EA, Mahoney J, Zhu XG, Delucia EH, Ort DR, Long SP (2004) Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under free-air carbon dioxide enrichment. Plant, Cell & Environment 27, 449–458.
| Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under free-air carbon dioxide enrichment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjs1ahtbs%3D&md5=37a7edb4f7c9514420bed616574e64f5CAS |
Roscher C, Thein S, Weigelt A, Temperton VM, Buchmann N, Schulze ED (2011) N2 fixation and performance of 12 legume species in a 6-year grassland biodiversity experiment. Plant and Soil 341, 333–348.
| N2 fixation and performance of 12 legume species in a 6-year grassland biodiversity experiment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjt1Wjtro%3D&md5=f924b64515f810849b2044daae8dddc4CAS |
Rosema A, Snel JFH, Zahn H, Buurmeijer WF, van Hove LWA (1998) The relation between laser-induced chlorophyll fluorescence and photosynthesis. Remote Sensing of Environment 65, 143–154.
| The relation between laser-induced chlorophyll fluorescence and photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Scharr H (2007) Optimal filters for extended optical flow. In ‘LNCS 3417 International Workshop on Complex Motion’. pp. 14–29. (Springer: Heidelberg, Germany)
Schmundt D, Stitt M, Jähne B, Schurr U (1998) Quantitative analysis of the local rates of growth of dicot leaves at a high temporal and spatial resolution, using image sequence analysis. The Plant Journal 16, 505–514.
| Quantitative analysis of the local rates of growth of dicot leaves at a high temporal and spatial resolution, using image sequence analysis.Crossref | GoogleScholarGoogle Scholar |
Schurr U, Walter A, Rascher U (2006) Functional dynamics of plant growth and photosynthesis - from steady-state to dynamics – from homogeneity to heterogeneity. Plant, Cell & Environment 29, 340–352.
| Functional dynamics of plant growth and photosynthesis - from steady-state to dynamics – from homogeneity to heterogeneity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xktlyltro%3D&md5=a3892a1a27df8880ea332409acac740fCAS |
Sharp RE, Silk WK, Hsiao TC (1988) Growth of the maize primary root at low water potentials. 1. Spatial-distribution of expansive growth. Plant Physiology 87, 50–57.
| Growth of the maize primary root at low water potentials. 1. Spatial-distribution of expansive growth.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cnhvVartg%3D%3D&md5=18e8b7e8bf24f782e1c2312f10afeb71CAS |
Silk WK (1992) Steady form from changing cells. International Journal of Plant Sciences 153, S49–S58.
| Steady form from changing cells.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Muchow RC (1999) Radiation use efficiency. Advances in Agronomy 65, 215–265.
| Radiation use efficiency.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Muchow RC, Ludlow MM, Leach GJ, Lawn RJ, Foale MA (1987) Field and model analysis of the effect of water deficits on carbon and nitrogen accumulation by soybean, cowpea and black gram. Field Crops Research 17, 121–140.
| Field and model analysis of the effect of water deficits on carbon and nitrogen accumulation by soybean, cowpea and black gram.Crossref | GoogleScholarGoogle Scholar |
Tambussi EA, Bort J, Araus JL (2007) Water use efficiency in C3 cereals under Mediterranean conditions: a review of physiological aspects. The Annals of Applied Biology 150, 307–321.
| Water use efficiency in C3 cereals under Mediterranean conditions: a review of physiological aspects.Crossref | GoogleScholarGoogle Scholar |
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant and Soil 341, 75–87.
| Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjt1Wjsrc%3D&md5=38d8909b7db73079ae5c15775eb4a5e4CAS |
Tsukamoto T, Nakanishi H, Uchida H, Watanabe S, Matsuhashi S, Mori S, Nishizawa NK (2009) 52Fe translocation in barley as monitored by a positron-emitting tracer imaging system (PETIS): evidence for the direct translocation of Fe from roots to young leaves via phloem. Plant & Cell Physiology 50, 48–57.
| 52Fe translocation in barley as monitored by a positron-emitting tracer imaging system (PETIS): evidence for the direct translocation of Fe from roots to young leaves via phloem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVehurg%3D&md5=2d34cbf507be093c5ccc32e6a1d9066fCAS |
Tyree MT, Ewers FW (1991) The hydraulic architecture of trees and other woody-plants. New Phytologist 119, 345–360.
| The hydraulic architecture of trees and other woody-plants.Crossref | GoogleScholarGoogle Scholar |
Ustin S, Gamon JA (2010) Remote sensing of plant functional types. New Phytologist 186, 795–816.
| Remote sensing of plant functional types.Crossref | GoogleScholarGoogle Scholar |
Ustin SL, Roberts DA, Gamon JA, Asner GP, Green RO (2004) Using imaging spectroscopy to study ecosystem processes and properties. Bioscience 54, 523–534.
| Using imaging spectroscopy to study ecosystem processes and properties.Crossref | GoogleScholarGoogle Scholar |
Van As H (2007) Intact plant MRI for the study of cell water relations, membrane permeability, cell-to-cell and long distance water transport. Journal of Experimental Botany 58, 743–756.
| Intact plant MRI for the study of cell water relations, membrane permeability, cell-to-cell and long distance water transport.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXislCrt70%3D&md5=b0c17eb97c52072e0bd195d71a9e3ab0CAS |
Van As H, Scheenen T, Vergeldt FJ (2009) MRI of intact plants. Photosynthesis Research 102, 213–222.
| MRI of intact plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVWhtrfM&md5=e5db09800781e336bc1a1cf30f3a9679CAS |
van Bel AJE (2003) Transport phloem: low profile, high impact. Plant Physiology 131, 1509–1510.
van der Mark W, Gavrila DM (2006) Real-time dense stereo for intelligent vehicles. IEEE Transactions on Intelligent Transportation Systems 7, 38–50.
| Real-time dense stereo for intelligent vehicles.Crossref | GoogleScholarGoogle Scholar |
van der Tol C, Verhoef W, Rosema A (2009) A model for chlorophyll fluorescence and photosynthesis at leaf scale. Agricultural and Forest Meteorology 149, 96–105.
| A model for chlorophyll fluorescence and photosynthesis at leaf scale.Crossref | GoogleScholarGoogle Scholar |
Walter A, Schurr U (2005) Dynamics of leaf and root growth: endogenous control versus environmental impact. Annals of Botany 95, 891–900.
| Dynamics of leaf and root growth: endogenous control versus environmental impact.Crossref | GoogleScholarGoogle Scholar |
Walter A, Spies H, Terjung S, Küsters R, Kirchgeßner N, Schurr U (2002) Spatio-temporal dynamics of expansion growth in roots: automatic quantification of diurnal course and temperature response by digital image sequence processing. Journal of Experimental Botany 53, 689–698.
| Spatio-temporal dynamics of expansion growth in roots: automatic quantification of diurnal course and temperature response by digital image sequence processing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitlCks7g%3D&md5=769fc2c47d14f0e8455facbbf69e73fcCAS |
Walter A, Silk WK, Schurr U (2009) Environmental effects on spatial and temporal patterns of leaf and root growth. Annual Review of Plant Biology 60, 279–304.
| Environmental effects on spatial and temporal patterns of leaf and root growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntFGls7c%3D&md5=062f2149d103f94dbb9da98bb926f4e6CAS |
Watt M, Silk WK, Passioura JB (2006) Rates of root and organism growth, soil conditions, and temporal and spatial development of the rhizosphere. Annals of Botany 97, 839–855.
| Rates of root and organism growth, soil conditions, and temporal and spatial development of the rhizosphere.Crossref | GoogleScholarGoogle Scholar |
Whitney SM, Houtz RL, Alonso H (2011) Advancing our understanding and capacity to engineer Nature’s CO2-sequestering enzyme, Rubisco. Plant Physiology 155, 27–35.
| Advancing our understanding and capacity to engineer Nature’s CO2-sequestering enzyme, Rubisco.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFags7c%3D&md5=7390d0e48339afd33350703cd16f0117CAS |
Windt CW, Vergeldt FJ, De Jager PA, Van As H (2006) MRI of long-distance water transport: a comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco. Plant, Cell and Environment 29, 1715–1729.
| MRI of long-distance water transport: a comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVWktLjO&md5=428adc24ff0c07a4e2bac27680144718CAS |
Windt CW, Gerkema E, Van As H (2009) Most water in the tomato truss is imported through the xylem, not the phloem: a nuclear magnetic resonance flow imaging study. Plant Physiology 151, 830–842.
| Most water in the tomato truss is imported through the xylem, not the phloem: a nuclear magnetic resonance flow imaging study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlSjsr%2FP&md5=423c7e07409037c33108d9ad47d9c268CAS |
Windt CW, Soltner H, van Dusschoten D, Blümler P (2011) A portable Halbach magnet that can be opened and closed without force: the NMR-CUFF. Journal of Magnetic Resonance (San Diego, Calif.) 208, 27–33.
| A portable Halbach magnet that can be opened and closed without force: the NMR-CUFF.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvF2ktg%3D%3D&md5=23992830f43cf7e4f9f6cc14654b4020CAS |
Yamashita N, Koike N, Ishida A (2002) Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status. Plant, Cell & Environment 25, 1341–1356.
| Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status.Crossref | GoogleScholarGoogle Scholar |
Zhu XG, Ort DR, Whitmarsh J, Long SP (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. Journal of Experimental Botany 55, 1167–1175.
| The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksVagsrk%3D&md5=10cc508b4449bc7920283bb9f930e140CAS |
Zhu XG, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology 61, 235–261.
| Improving photosynthetic efficiency for greater yield.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnslSjsL8%3D&md5=07486c7da91af9cf1d6ece0377e33664CAS |