Partitioning hydraulic resistance in Sorghum bicolor leaves reveals unique correlations with stomatal conductance during drought
Troy W. Ocheltree A C D , Jesse B. Nippert B , Mary Beth Kirkham C and P. Vara V. Prasad CA Department of Forest Resources, University of Minnesota, 1530 Cleveland Avenue N., St. Paul, MN 55108, USA.
B Division of Biology, Kansas State University, 116 Ackert Hall, Manhattan, KS 66506, USA.
C Department of Agronomy, Kansas State University, 2004 Throckmorton Hall, Manhattan, KS 66506, USA.
D Corresponding author. Email: ochel005@umn.edu
Functional Plant Biology 41(1) 25-36 https://doi.org/10.1071/FP12316
Submitted: 25 October 2012 Accepted: 31 May 2013 Published: 30 July 2013
Abstract
The hydraulic architecture of leaves represents the final path along which liquid water travels through the plant and comprises a significant resistance for water movement, especially for grasses. We partitioned leaf hydraulic resistance of six genotypes of Sorghum bicolor L. (Moench) into leaf specific hydraulic resistance within the large longitudinal veins (r*LV) and outside the large veins (r*OLV), and correlated these resistances with the response of stomatal conductance (gs) and photosynthesis (A) to drought. Under well-watered conditions, gs was tightly correlated with r*OLV (r2 = 0.95), but as soil moisture decreased, gs was more closely correlated with r*LV (r2 = 0.97). These results suggest that r*OLV limits maximum rates of gas exchange, but the ability to efficiently move water long distances (low r*LV) becomes more important for the maintenance of cell turgor and gas exchange as soil moisture declines. Hydraulic resistance through the leaf was negatively correlated with evapotranspiration (P < 0.001) resulting in more conservative water use in genotypes with large leaf resistance. These results illustrate the functional significance of leaf resistance partitioning to declining soil moisture in a broadly-adapted cereal species.
Additional keywords: drought tolerance, leaf hydraulic conductance, stomatal conductance, plant anatomy.
Introduction
Leaves provide the direct link between water loss to the atmosphere and carbon gain by plants, but the dynamic resistance to water movement through leaves responds to a range of environmental conditions. The hydraulic resistance of the whole leaf (r*Leaf) is tightly correlated with maximum rates of gas exchange across a broad range of species (Brodribb et al. 2007), but also changes temporally in response to leaf hydration status (Brodribb and Holbrook 2003; Kim and Steudle 2007; Heinen et al. 2009) and incident light (Tyree et al. 2005; Scoffoni et al. 2008). These temporal responses of r*Leaf can be mediated through cavitation events within the leaf xylem (Nardini et al. 2003; Trifilò et al. 2003; Johnson et al. 2012), cell collapse of the xylem elements (Cochard et al. 2004a; Brodribb and Holbrook 2005; Blackman et al. 2010) or changes in aquaporin regulation after water leaves the vasculature (Cochard et al. 2007). Taken together, these results illustrate the varying function of individual components of r*Leaf on the rates of leaf gas exchange.
Leaf resistance in dicot leaves has been previously partitioned into xylary and extra-xylary components that range widely across species; the relative proportion of these two resistances in leaves range from 26 to 80% of total leaf resistance (Martre et al. 2001; Zwieniecki et al. 2002; Cochard et al. 2004b; Sack et al. 2004; Nardini and Salleo 2005). Across a range of species and environmental conditions, both xylary and extra-xylary resistance have been linked to maximum rates of gas exchange (Sack et al. 2002; Sack and Frole 2006; Brodribb et al. 2007), but the role of these two leaf resistances for regulating stomatal responses to decreasing soil moisture has not been investigated. Furthermore, this type of hydraulic partitioning has not been investigated in grasses, in which the majority of the aboveground pathway for water movement occurs within leaves (either the leaf sheath or leaf blade) of most species. Detangling leaf resistances to water movement is vital to a better understanding of hydraulics in this growth form. Long-distance axial transport of water along monocot leaves occurs within the large longitudinal veins, and the local distribution of water to cells occurs via small longitudinal and transverse veins (Altus and Canny 1985; Altus et al. 1985). To partition leaf hydraulic resistance in monocots into the two most significant functional components, we classified resistance to long-distance water movement within the large longitudinal veins (r*LV) and resistance outside the large longitudinal veins (r*OLV) as water is distributed locally through small longitudinal and transverse veins and leaf tissue outside the vascular bundles.
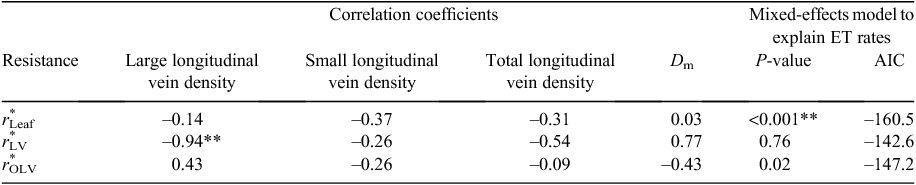
When component resistances act in series within a network, the largest resistance should be most limiting to the movement of water (Meinzer 2002). When fully hydrated, the type of leaf hydraulic tissue with the greatest resistance varies between species (Sack et al. 2005), but for many of the species investigated the major resistance to water movement is outside the xylem (Cochard et al. 2004b; Gascó et al. 2004; Nardini and Salleo 2005; Mott 2007). Coupled measurements of partitioned leaf resistances and gs are rare, and as such, it is unknown whether maximum gs correlates with r*OLV for previously measured plant species, as theory would suggest. A strong correlation has been shown between r*Leaf and the distance from vascular bundle to stomata (Dm) across a broad range of species (Brodribb et al. 2007) and within grass leaves (Kodama et al. 2011; Ocheltree et al. 2012). Dm is an indirect measure of extra-xylary resistance but it is not clear whether Dm directly affects hydraulic resistance or whether it is indirectly related to another component of leaf hydraulic resistance. Therefore, further investigation is required to relate leaf hydraulic resistance to the control of maximum gs in grasses.
As soil moisture declines and becomes limiting to plant growth, gs responds to changes in water potential in the immediate vicinity of the stomata (Buckley 2005) or internal water vapour concentration (Peak and Mott 2011) to maintain hydration of plants cells. Plants often maintain near-maximum rates of gs until leaf water potential reaches a threshold (Girma and Krieg 1992; Tardieu and Simonneau 1998), at which point gs is reduced to maintain leaf water potential above the point when cavitation would cause catastrophic loss of hydraulic function while plants are transpiring (Sperry et al. 1998). Plants with low hydraulic resistance to long-distance transport should be able to minimise the water potential gradient from the soil matrix to leaf surface and maintain relatively high gs (Tyree and Zimmerman 2002), which has been identified among woody species (Meinzer and Grantz 1990; Meinzer et al. 1995; Hubbard et al. 2001) and across developmental stages (Saliendra et al. 1995) when soil moisture was limited. The correlation between xylem resistance within leaves (rather than woody tissue) and gs has not been tested in grass species.
If the tissue with the greatest resistance controls the rate of water movement through a leaf, then maximum stomatal conductance should correlate with that tissue, but the role of hydraulic resistance as a regulator of leaf gas exchange in grasses is under-studied. The goal of this study was to assess the relationship between stomatal conductance and the resistance of different tissues within leaves of six different genotypes of Sorghum bicolor that vary in drought tolerance. We hypothesised that: (i) maximum rates of gas exchange will be negatively correlated with r*OLV under well-watered conditions, but (ii) as water becomes limiting to plant growth, rates of gas exchange will be negatively correlated with r*LV to minimise the water potential gradient from soil to leaf. As such, genotypes with low resistance within the xylem will be able to maintain higher rates of gas exchange when soil moisture is limiting. Furthermore, we hypothesised that (iii) genotypes with greater r*OLV will exhibit a more ‘water-conservative’ growth strategy, having lower initial rates of gas exchange but maintaining these rates further into a drought treatment.
Materials and methods
Plant material
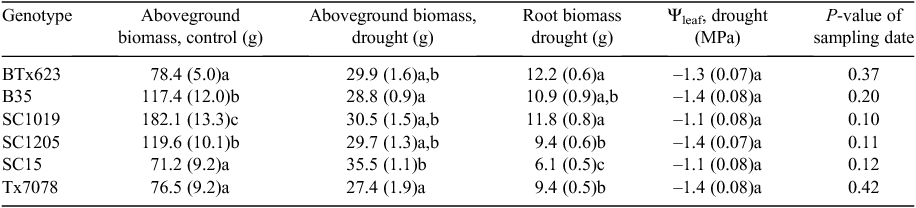
Six genotypes of Sorghum bicolor L. (Moench) were selected for this study covering a range of drought tolerance: SC1019, SC1205, SC15, Tx7078, BTx623 and B35. The study was divided into two sections: (1) the first, in which we measured leaf hydraulic resistance for each genotype on one group of plants, and (2) the second in which leaf gas exchange was measured in response to drought on a second group of plants. In both studies, two seeds were planted in 20 L pots filled with potting soil (Metro-Mix 360, Sun-Gro Horticulture Canada Ltd, Vancouver, BC, Canada) and were thinned to one individual per pot after germination. Plants were supplied with a controlled-release fertiliser (Scotts-Sierra Horticultural Products Co., Marysville, OH, USA) and watered daily. When germinating plants for the determination of hydraulic resistance, two replicates for each genotype were planted each week so that plants would develop in stages to allow sampling of replicates at the same developmental stage; hydraulic resistance was measured on the 5th or 6th leaf of each plant. Plants were grown in a greenhouse where maximum daily PAR was between 800 and1200 μmol m–2 s–1 and daily temperatures were maintained between 22 and 26°C. For the drought response experiment, eight replicates of each genotype were planted at the same time and grown under well-watered conditions until the 5th leaf had fully expanded, then water was withheld to initiate the drought treatment.
Hydraulic resistance
Hydraulic resistance was measured using a hydrostatic gradient to force water through the sample (Sperry et al. 1988) with a custom chamber designed for large grass leaves. The chamber was constructed to accommodate the large leaves of sorghum and allowed us to quantify both axial resistance within the large longitudinal veins (r*LV), and the resistance to water movement locally within the leaf outside the large veins (r*OLV). The hydraulic chamber consisted of two compartments: the first was a small ‘pressurised compartment’ where the basal cut section of a leaf was affixed and water forced into the leaf, and the second was a ‘collection compartment’ where water flowing through the leaf was collected and diverted to a balance through 3.2 mm internal diameter tubing (Bev-a-Line, Thermoplastic Processes, Georgetown, DE, USA). De-ionised and degassed water was forced through the leaf at ~20 kPa and was collected on a balance (±0.0001 g, Pioneer PA214, Ohaus Corporation, Pine Brook, NJ, USA) connected to a datalogger (CR1000, Campbell Scientific Inc., Logan, UT, USA) to monitor the rate of water flow through the system at 5 s intervals. Here we considered 20 kPa likely to be insufficient to displace embolisms, and estimates of resistance likely to include any dysfunction produced by cavitation. We minimised the impact of cavitation on our measurements, however, by ensuring the soil was at pot-holding capacity and the plants were kept in a darkened chamber for ~12 h before the time of the hydraulic measurements. Pressure transducers (Model 68075, Cole-Parmer Instrument Co., Vernon Hills, IL, USA) were placed on both inlet and outlet tubing to measure the pressure gradient across the leaf and a thermocouple measured water temperature in the collection compartment. Once the flow through the leaf reached steady-state (normally ~15–30 min), 5 min of data was recorded by the datalogger. The background flow was determined by equilibrating the pressure on each side of the leaf so that no pressure gradient existed across the leaf segment and the flow was measured for 5 min (Sperry and Hacke 2002). This was performed before and after each measurement, and the average of the two measurements was subtracted from the pressurised flow and the result multiplied by the pressure gradient to yield hydraulic resistance (MPa s mmol–1) not yet normalised by leaf area or length. The system used to measure hydraulic resistance was cleaned with a mild bleach solution and then rinsed repeatedly with de-ionised water each morning before measurements were initiated.
The most recently matured leaf (5th or 6th leaf) was cut from the plant under water and was placed in the hydraulic chamber so the longitudinal midpoint of the leaf was centred in the ‘collection compartment’ (as described above). The basal portion of the leaf was re-cut with a razorblade and ~2 cm of the leaf was immediately sealed in the ‘pressurised compartment’ of the chamber. For determination of leaf hydraulic resistance (rLeaf), ~50 cm2 of leaf area was enclosed in the ‘collection compartment’ that was connected to a reservoir on the balance. The leaf area in the ‘collection compartment’ and the remainder of the leaf distal to the chamber were kept submerged in the water bath during the measurement. In this way, water flowed into the basal end of the leaf and travelled through both xylary and extra-xylary pathways to the collection chamber allowing quantification of total leaf hydraulic resistance, not yet normalised by leaf area (rLeaf, MPa s mmol–1). The collection compartment was illuminated at 2000 μmol m–2 s–1 PAR using a fibre-optic light source (FL-150, Meiji Techno Co., Saitama, Japan), as rLeaf of the genotypes studied were sensitive to incident light (data not shown). Incident PAR was estimated using a quantum sensor (Li-190, Li-Cor Inc., Lincoln, NE, USA) sealed in an acrylic chamber (made of acrylic identical to the hydraulic chamber) submerged under water and placed at the same level as the leaf.
Following the measurement of rLeaf, the collection compartment was opened and a transverse cut was made across the leaf to remove most of the leaf area and expose the xylem for determination of axial hydraulic resistance. A fresh transverse cut was also made on the basal end of the leaf and then both chambers were re-sealed, leaving a leaf segment ~10 cm long for determination of rLV. These cuts removed the resistance of the smaller longitudinal veins, transverse veins and extra-xylary tissue from the measurement. Flow rate through the xylem proceeded as previously described. To ensure there were no vessel elements longer than our leaf section, we removed water from the ‘pressurised chamber’ and forced air through the leaf and made sure no air bubbles were passing through the leaf section. Both rLV and rLeaf represent raw resistance values and are not normalised by leaf area or length. Following hydraulic resistance measurements, the leaf area of the entire leaf was measured so that rLeaf, rLV and rOLV could be normalised by leaf area.
We assumed rLeaf consisted of two resistances in series, rLV (MPa s mmol–1) and rOLV (MPa s mmol–1), which allowed rOLV to be calculated based on direct measurements of rLeaf and rLV (Eqn 1) using an electrical analogue approach. Thus, rLeaf and rOLV were normalised by the amount of leaf area (LA, m2) inside the collection chamber (Eqns 2 and 3 respectively) and rLV was normalised by both the length of the leaf section measured (lseg, m) and the leaf area distal (LA, m2) to this section (Eqn 4, MPa mmol–1 s m):




Symbols including ‘*’ represent data that has been normalised by leaf area. In the case of r*LV, it has also been normalised by segment length. When normalised in this manner, r*LV remains constant along leaf blades in a range of grass species (Ocheltree et al. 2012).
Anatomy
A small section of each leaf, taken from the longitudinal centre, was placed in a vial of 10% formalin/5% acetic acid/50% ethanol/35% DI H2O, vacuum infiltrated overnight (Ruzin 1999) and stored at 4°C until the tissue could be embedded with parafilm and stained with toluidine blue for microscopic analysis (Kansas State University Histology Laboratory, College of Veterinary Medicine, Manhattan, KS, USA). Digital images were taken of each leaf, from the midrib to the outer edge of one-half of the leaf (Leica DFC 290; Leica Microsystems GmbH, Wetzlar, Germany) at ×20 magnification using a light microscope (Leica DM1000; Leica Microsystems GmbH). The wide leaves of this species required that multiple images be taken to cover this entire region. Large longitudinal veins were identified by the presence of large meta-xylem vessel elements and the remaining veins were categorised as other smaller veins. Both of these vein classes were counted in the digital images, and used to calculate vein density. We assumed that no veins terminated within 1 mm of the leaf section used for microscope analysis and calculated vein density (mm mm–1) by multiplying the number of veins by 1 mm to get the length of each vein size class and divided this by leaf area. The diffusional distance from vascular bundle to the nearest stomata (Dm) was estimated as described by Ocheltree et al. (2012) by calculating the hypotenuse between the vertical and horizontal distances from the edge of the vascular bundle to stomate.
Gas exchange
Gas-exchange measurements were made on the second set of plants during the drought treatment. After plants had five mature leaves, water was completely withheld to simulate a drought. Gas-exchange rates were measured (Li-6400 Li-Cor Inc.) on the centre of the leaf, as rates of gas exchange vary with leaf position in this species (TW Ocheltree, unpubl. data). All measurements were made between 1100 and 1500 hours with clear skies to maximise light availability in the greenhouse and best estimate maximum rates of gas exchange for that day. Measurements were repeated every 3–4 days to capture the response of leaf-level gas-exchange rates to drying soils. Prior to gas-exchange measurements, the mass of each potted plant was measured (±50 g, model CTB-600, Citizen Scales, Mumbai, India) so we could determine soil water content and rates of evapotranspiration (ET). Gas-exchange measurements were continued until gs < 0.05 mol m–2 s–1 and the plants were severely wilted, which ranged from 21 to 30 days for the individuals and genotypes measured.
Biomass production
After the drought experiment was complete, plants were harvested and divided into above and belowground components. Soil was washed from the roots with a low-pressure spray nozzle and the dislodged soil was collected in a large bucket to sit overnight, allowing the soil to separate gravimetrically. After ~24 h the majority of the water was siphoned off the top and the soil was dried at 60°C in a shallow pan. Several soil samples were weighed every day during the drying process and it was determined that the soils were >99% dry by the end of 7 days; all subsequent soil samples were dried for 7 days and then weighed (±0.01 g, Pioneer PA3102, Ohaus Corporation, Pine Brook, NJ, USA). Above- and belowground plant biomass was dried at 60°C for 48 h and then weighed (±0.0001 g, Pioneer PA214, Ohaus Corporation). Soil water content (SWC) was determined on a mass basis from the measurements of the potted plants during the experiment and the dried soil:

where mwet (kg) is the mass of the potted plant during the experiment and mdry (kg) is the mass of the dry soil. The mass of the plant was included in the measurement of mwet, so changes in SWC based on our measurements reflect changes in both soil moisture content and plant biomass. The final plant dry mass was <2% of the total soil dry mass and so changes in SWC based on Eqn 5 were mainly driven by changes in soil moisture rather than changes in plant biomass. As previously mentioned, the soil used was a horticultural potting soil, and so SWC values presented are much higher than would be expected in field conditions.
Leaf water potential
Leaf water potential (ΨLeaf) was measured throughout the drought experiment using thermocouple psychrometers (70 series, JRD Merril, Logan, UT, USA) attached to a CR7 datalogger (Campbell Scientific Ltd, Logan, Utah, USA). Leaf water potential measurements were made immediately following gas-exchange measurements by removing a 5 mm leaf disc using a disposable biopsy punch (Integra Miltex, York, PA, USA). The leaf disc was immediately placed in the psychrometer chamber, which was then placed in a 25°C water bath to equilibrate for 1 h before determining ΨLeaf. The measurement of wet-bulb depression was made according to Comstock (2000) and converted to ΨLeaf based on the calibration of NaCl standards measured in the same manner as leaf discs.
Statistical analyses
All statistical analyses were performed using the R open-source software package (R Development Core Team 2011). To identify differences in leaf resistances between genotypes, multiple pairwise comparisons were made using the ‘Holm’ correction for multiple comparisons and significant differences were determined at the P < 0.05 level. Standard linear regression was used to identify correlations between leaf resistances, gs and leaf anatomy. When the relationship between gs and SWC was plotted, three different stages were apparent; constant maximum rates of gs when soil moisture was high, a linear decrease in gs as soil moisture decreased, and then a steeper decline in gs when soil moisture was extremely low. We identified the breakpoints between these three stages using a piecewise regression (‘segmented’ package in R), and averaged rates of gas exchange within each stage of the SWC × gs relationship. A Pearson’s correlation was calculated between the leaf resistances measured and leaf anatomy and considered significant at the P < 0.05 level. The rate that SWC decreased was determined by calculating the slope of the line between SWC and sampling date. In order to test for correlations between leaf resistances and ET but account for the effect of increasing plant size on ET, we used a mixed-effects model with ‘sampling date’ as a random effect in the model.
Results
Hydraulic resistance
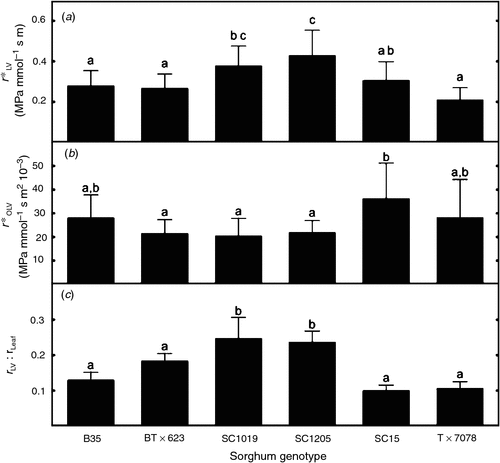
We found significant differences among the six genotypes of Sorghum bicolor in both r*LV and r*OLV (Fig. 1). Results show that r*LV ranged from 0.21 to 0.43 MPa mmol–1 s m across the six genotypes (Fig. 1a), which highlights the potential variability within a single species. Further, r*OLV ranged from 0.020 to 0.036 MPa mmol–1 s m2 (Fig. 1b), but few significant differences among genotypes were present owing to the large variability within each genotype, which incorporates the uncertainty associated with measurements of both rLeaf and rLV (Eqn 1). The proportion of rLeaf in the large longitudinal veins ranged between 0.10 and 0.25 for all genotypes (Fig. 1c), which means that 75–90% of leaf resistance was outside the large longitudinal veins for the genotypes studied.
|
Gas exchange
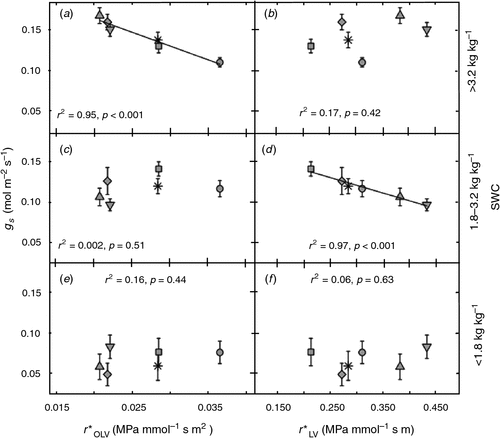
Stomatal conductance correlated with different components of leaf hydraulic resistance as soil moisture declined (Fig. 2). Using data from all the genotypes, we identified three different phases in the relationship between SWC and gs (data not shown); a steady maximum rate of gs when soil moisture was high (SWC >3.2 kg/kg), a linear decline in gs as soil moisture became limiting to gs (1.8 < SWC <3.2 kg kg–1), followed by a more rapid decline in gs when soil moisture was extremely low (SWC <1.8 kg kg–1). When SWC was high (>3.2 kg kg–1), gs was inversely related to r*OLV (Fig. 2a, P < 0.001) but was not significantly correlated with r*LV (Fig. 2b, P = 0.42). As SWC decreased to the range of 1.8–3.2 kg kg–1, the gs of most genotypes declined and was negatively correlated with r*LV (Fig. 2d, P < 0.001) but not r*OLV (Fig. 2c, P = 0.51). When soil moisture dropped below 1.8 kg kg–1, there was no correlation between either r*OLV or r*LV (Fig. 2e, f).
|
Evapotranspiration (ET) increased throughout the experiment as the demand for water increased with the growing plant canopy (data not shown). To investigate the correlation between r*Leaf and ET, we first accounted for the increasing ET by including ‘sampling date’ as a random effect in a mixed-effects model. After accounting for the temporal increase in ET there was a negative correlation between r*Leaf and ET (Table 1), and genotypes with greater leaf resistance used water more conservatively (ET g day–1 = rLeaf × 46.6 + 87.0). A 50% increase in r*Leaf would only result in a 9% increase in water savings per day, but this amount would be important over the course of a growing season. Data showed r*OLV was a significant predictor of ET (P = 0.02), but the integration of r*OLV and r*LV in r*Leaf provided a better fit for the mixed-effects model explaining ET (Table 1). For example, genotype SC15 had the largest r*Leaf but had an ET 20% less than the genotype with the lowest leaf resistance (BTx623) despite accumulating more aboveground biomass during the experiment (Table 2).
Soil moisture also decreased more rapidly in plants with low r*Leaf (Fig. 3). A linear relationship was moderately significant (P = 0.07), but the data suggested a non-linear relationship. In order to fit a curve to the data, both r*Leaf and the rate of SWC depletion were scaled between 0 and 1. When scaled in this manner, an exponential decay function fit the data closely (Fig. 3, inset). This supports the ET data, described above, suggesting that genotypes with greater r*Leaf used water more conservatively.
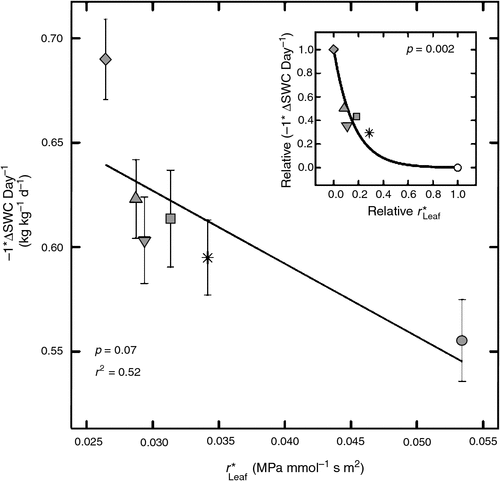
|
Leaf water potential
All six genotypes studied had similar ΨLeaf values throughout the experiment (Table 2), and the average ΨLeaf was –1.3 MPa across all genotypes and sampling periods. There were no significant difference between genotypes in ΨLeaf for any of the sampling periods, and there was no significant difference in ΨLeaf between sampling periods within any genotype. ΨLeaf was not determined on the last sampling date when gs ≤ 0.05 mol m–2 s–1. These results suggest that all genotypes regulated gs to help maintain ΨLeaf at similar values across a range of soil water availabilities.
Biomass production
Few differences in final biomass production were found between genotypes when grown under drought (Table 2), but the genotype with the greatest resistance in the leaves (SC15), did have the highest aboveground biomass (Table 2). Aboveground biomass in the control treatment showed greater variability but was not correlated with either r*LV or r*OLV (data not shown). Root biomass in the drought experiment varied by genotype (Table 2) and was correlated with r*Leaf (Fig. 4). Plants with greater resistance in their leaves (and lower gs) had less root biomass (Fig. 4a) and smaller root : shoot ratios (Fig. 4b). Root biomass and root : shoot ratio were still highly correlated with r*Leaf when a Spearman’s rank correlation was calculated (ρ = –0.82 and –0.77 respectively), suggesting that the genotype with high r*Leaf was not driving the correlations.
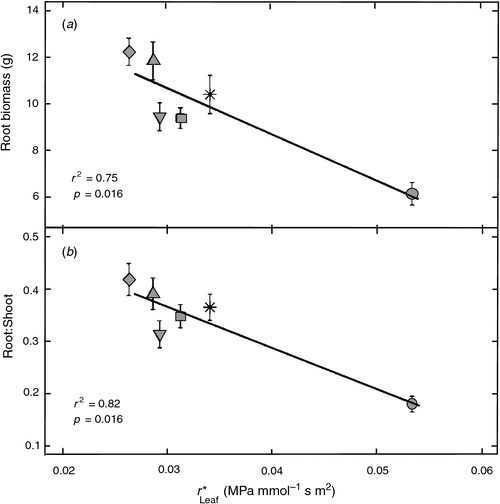
|
Anatomy
Large longitudinal vein density was correlated with r*LV (Fig. 5a), suggesting the bulk of axial water movement was in this vein order. Small and total longitudinal vein density did not correlate with any measure of leaf resistance. The distance of water movement from the vascular bundle to stomata (Dm) varied little among genotypes, and only ranged from 39.1–47.9 μm. Dm was not significantly correlated with r*OLV (Fig. 5b), or any other measurement of leaf resistance (Table 1).
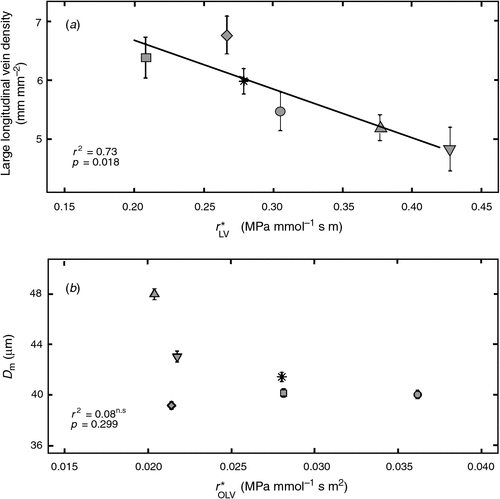
|
Discussion
The partitioning of hydraulic resistance within leaves has important functional consequences for understanding plant responses to declining soil moisture. When resistances were calculated for a fully-saturated leaf, the resistance outside the large longitudinal veins (r*OLV) was tightly correlated with maximum rates of gas exchange when soil moisture was readily available. As soil moisture decreased, the axial resistance within the large longitudinal veins of grass blades (r*LV) was a better predictor of gas exchange rates. Because these two resistances influenced stomatal conductance under different soil moisture conditions, the integration of these resistances in r*Leaf was the best predictor of whole-plant water use (estimated by ET) over the course of the experiment.
Previous work partitioning leaf resistances has separated the resistance of water movement within the xylem from the resistance outside the xylem (Sack and Holbrook 2006). The partitioning of resistances done here differs from previous work in that we partitioned r*Leaf into: long-distance resistance within the large longitudinal veins (r*LV) from the resistances imposed by other sources of local water distribution (r*OLV) (Altus and Canny 1985; Altus et al. 1985). Measurement of r*OLV is likely to include the hydraulic resistance within the small longitudinal, transverse veins and the resistance of the mesophyll. However, we used a lower pressure during our r*Leaf measurements than previous studies, so it is possible that water was not forced through the smallest veins, thus excluding water movement through some veins and the mesophyll during our measurements. Our r*Leaf values are within values recently reported for grasses (Holloway-Phillips and Brodribb 2011a, 2011b) and other crop species (Tsuda and Tyree 2000), however, the possibility exists that we overestimated r*Leaf and r*OLV. Within a single species we found r*OLV to vary from 75 to 90% of total leaf resistance, which is within the range reported when partitioning xylary from extra-xylary resistance in leaves (Zwieniecki et al. 2002; Cochard et al. 2004b; Sack et al. 2004, 2005; Nardini and Salleo 2005). However, the per cent of whole-leaf resistance in r*OLV is confined to the high end of the values reported, which is probably because there is less variability within this genotype than among species.
The greatest resistance to water movement occurred outside the large longitudinal veins in all the genotypes studied (Fig. 1c), and this source of resistance should be the rate-limiting step in the movement of water through these plants when water was not limiting. Consistent with this reasoning, we found r*OLV to be tightly correlated with gs when soil moisture was readily available (Fig. 2a). Previous work has shown indirect estimates of resistance outside the xylem correlated with maximum rates of gas exchange, as the distance from vascular bundle to stomata (Dm) was correlated with gs within grass blades (Ocheltree et al. 2012) and among dicot leaves (Brodribb et al. 2007). We found no correlation between Dm and gs in this study (Fig. 4b) and other recent work also failed to find this relationship among closely related species (Nardini et al. 2012). The range of Dm values among the six genotypes of Sorghum bicolor studied here was small (39.1–47.9 μm) compared with previous work (~100–1200 μm, Brodribb et al. 2007). Therefore, it is possible that Dm is indirectly related to leaf resistance and captures the variability across large gradients in leaf structure, but among species with similar leaf structure other mechanisms may control maximum hydraulic conductance through the leaf (Nardini et al. 2005; Sadok and Sinclair 2010).
As water becomes limiting to plant growth and gs is reduced in order to maintain the hydration of plant cells, the ability to efficiently transport water long distances should minimise pressure gradients within plants. All six genotypes included in this study maintained midday ΨLeaf at approximately –1.3 MPa as soil moisture declined (Table 2), which falls within the range of values previously reported for this species (Ackerson et al. 1977; Jones and Turner 1978). This relatively constant ΨLeaf throughout the drought treatment suggests that gs decreased to maintain the water status of the leaves. At similar ΨLeaf values among genotypes, we expected genotypes with low r*LV to minimise the pressure gradients along the leaves and maintain higher rates of gs at comparable levels of soil moisture and vapour pressure deficit. Our data support this idea (Fig. 2d), as there was a tight correlation between gs and r*LV when soil moisture was limiting that did not exist under well-watered conditions (Fig. 2b). Stomatal conductance of Helianthus annuus cv. Margot has also been shown to correlate with the resistance in the leaf xylem when plants were grown at different levels of soil moisture availability (Nardini and Salleo 2005). These results emphasise the importance of leaf xylem resistance in controlling rates of leaf gas exchange during periods of water limitation.
It should be noted that our measurements of leaf hydraulic resistance were made on well-watered plants, which were then related to gas-exchange measurements during a drought treatment. Leaf hydraulic resistance responds to leaf water status through, at least, two mechanisms: reduced conductance due to xylem dysfunction (Trifilò et al. 2003; Cochard et al. 2004a; Marenco et al. 2006), or changes in aquaporin transport as water moves through the leaf mesophyll (Kim and Steudle 2007). Hydraulic resistance through the xylem changes on a diurnal basis as vessel elements cavitate and refill in response to changes in leaf hydration status (Marenco et al. 2006) and has recently been shown to correlate closely with changes in r*Leaf (Johnson et al. 2012). The leaf water potential of the genotypes in our study did not change significantly in response to the drought treatment (Table 2); instead gs decreased keeping the leaf water potential relatively constant during our experiment. This suggests that diurnal patterns of cavitation events or changes in aquaporin regulation due to changes in hydration status would have had consistent affects on leaf hydraulic resistance throughout most of our experiment. As such, the resistance partitioning performed on well-watered plants should have remained relevant to leaf function as the plants experienced drought.
We found significant correlations between leaf structure and hydraulic resistance within the xylem of the genotypes we studied. The number of large longitudinal veins correlated strongly with r*LV across these six genotypes (Fig. 5a). The dimensions and number of vessel elements in the large longitudinal veins were similar across genotypes (data not shown), so differences in r*LV between genotypes resulted from the absolute number of large longitudinal veins present in the leaf (Fig. 5a). This matches model simulations of leaves where increasing major vein density led to a linear decrease in xylem resistance (McKown et al. 2010). Decreasing r*LV through changes in the number of vascular bundles rather than the size of vessel elements is expensive to plants as cost of xylem construction is fairly high (McCulloh et al. 2003). We found no significant correlations between any anatomical characteristics (Dm or vein density) and r*OLV, which suggests that the hydraulic resistance outside the large longitudinal veins may have been controlled by other mechanisms. For example, we did not account of the density of transverse veins, which could also account for additional variation in r*OLV.
It has been suggested that plants with high r*Leaf exhibit a conservative water-use strategy (Sinclair et al. 2008), leaving more water for biomass production later in the growing season. Consistent with this idea, we found r*Leaf was correlated with ET throughout the experiment among the genotypes we studied (Table 1). This relationship was likely driven by differences in the transpiration component of ET, as evaporation from the soil surface was unlikely to differ much between individuals due to similarity in canopy structure above the soil surface. Differences in ET could also have resulted from differences in biomass between genotypes, as larger plants with greater leaf area used more water independent of r*Leaf. This explanation is unlikely though, as genotypes with the lowest ET rates also had the greatest aboveground biomass at the end of the experiment (e.g. genotype SC15). The best explanation is that slower ET rates were directly related to low r*Leaf, as genotypes with the greatest leaf resistance had the slowest soil moisture depletion rates (Fig. 3).
If a water conservation growth strategy leaves more water for biomass production later into a drought, we would have expected the genotypes with high r*Leaf to have higher final biomass production; this was not the case for the genotypes in this study. This may have been because our experimental design, as we were not simulating field conditions but exposing plants to an extreme drought over a relatively short period of time while confining roots to relatively small volumes of soil. Comparing these genotypes in situ would help determine if a ‘water conserving’ strategy resulting from high r*Leaf is advantageous to biomass production. The lower demand for water with increasing r*Leaf did correlate with differences in biomass partitioning between genotypes. Across all six genotypes the root : shoot ratio was significantly correlated with the resistance of leaves (Fig. 4b). High resistance in the leaf resulted in less demand for water with greater resource allocation aboveground. Because we only measured biomass at the end of the experiment, we cannot be certain that the biomass partitioning was a response to the drought treatment or whether this represents inherent differences among genotypes. In either scenario, the strong correlation between r*Leaf and root : shoot ratios suggests a link between hydraulic architecture and plant growth strategy that demands further in situ investigations.
In natural ecosystems it is unclear when a ‘water conserving’ strategy would be competitively advantageous for plants; dominant species are often inefficient users of water (DeLucia and Schlesinger 1991), so large r*OLV that limits water use may not be a successful competitive strategy in many systems. In mesic systems where drought is typically moderate, minimising r*OLV and maximising the efficiency of water transport through the xylem to maintain hydrated cells should be advantageous. Indeed, within many ecosystems fast-growing species often have the highest stem hydraulic conductance (Brodribb and Feild 2000; Markesteijn et al. 2011). In xeric systems where competition for water may not be as important as tolerating long periods of drought, conserving water may be advantageous to growth and survival (Fernández and Reynolds 2000). Under this scenario, high r*OLV in leaves may facilitate whole-plant water conservation despite the hydraulic architecture of the remainder of the plant. The ability to tolerate low soil moisture and low leaf water potentials would be more important in xeric systems, and likely reflects characteristics of the individual vessel elements that limit cavitation (Wheeler et al. 2005; Hacke et al. 2006; Blackman et al. 2010) and major vein density (Scoffoni et al. 2011). Investigating how leaf resistances are partitioned in a widely distributed group of grasses would provide new insights into how leaf resistances contribute to plant distributions across the landscape.
Our results show the correlation between leaf hydraulic resistance and the response of leaf-level gas exchange to drying soils in S. bicolor. High resistance outside the large longitudinal veins correlated with low rates of gas exchange when soil moisture was readily available to plants, but these genotypes of S. bicolor conserved water and were able to maintain higher rates of gas exchange further into the drought treatment. Furthermore, the efficient transport of water within the large longitudinal veins sustained leaf-level gas exchange under moderate drought. Future work should focus on understanding leaf function under severe drought, and measure leaf resistance partitioning across a broader range of species. Our results suggest that the partitioning of hydraulic resistance within leaves provides a framework to assess mechanisms of plant growth under a range of soil moisture conditions.
Acknowledgements
We thank George Mahama, Raymond Mutava, and Jeff Hartman for their technical support during this study, and Dr Kendra McLauchlan for the use of laboratory facilities. Financial support was provided, in part, by the Kansas Technology Enterprise Corporation, The Konza Prairie LTER (DEB-0823341), and the K-State Center for Sorghum Improvement, and the Kansas Agriculture Experiment Station, contribution number 13–156-J.
References
Ackerson RC, Krieg DR, Miller TD, Zartman RE (1977) Water relations of field grown cotton and sorghum: temporal and diurnal changes in leaf water, osmotic, and turgor potentials. Crop Science 17, 76–80.| Water relations of field grown cotton and sorghum: temporal and diurnal changes in leaf water, osmotic, and turgor potentials.Crossref | GoogleScholarGoogle Scholar |
Altus D, Canny M (1985) Water pathways in wheat leaves. 1. The division of fluxes between different vein types. Australian Journal of Plant Physiology 12, 173–181.
| Water pathways in wheat leaves. 1. The division of fluxes between different vein types.Crossref | GoogleScholarGoogle Scholar |
Altus D, Canny M, Blackman D (1985) Water pathways in wheat leaves. 2. Water-conducting capacities and vessel diameters of different vein types, and the behavior of the integrated vein network. Australian Journal of Plant Physiology 12, 183–199.
| Water pathways in wheat leaves. 2. Water-conducting capacities and vessel diameters of different vein types, and the behavior of the integrated vein network.Crossref | GoogleScholarGoogle Scholar |
Blackman CJ, Brodribb TJ, Jordan GJ (2010) Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytologist 188, 1113–1123.
| Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms.Crossref | GoogleScholarGoogle Scholar | 20738785PubMed |
Brodribb TJ, Feild TS (2000) Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests. Plant, Cell & Environment 23, 1381–1388.
| Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests.Crossref | GoogleScholarGoogle Scholar |
Brodribb TJ, Holbrook NM (2003) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology 132, 2166–2173.
| Stomatal closure during leaf dehydration, correlation with other leaf physiological traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmsVantbc%3D&md5=5e37abae6f351712a8923a2cc0de35c9CAS | 12913171PubMed |
Brodribb TJ, Holbrook NM (2005) Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiology 137, 1139–1146.
| Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXislOqs7k%3D&md5=55177ec044765c553099af456f809236CAS | 15734905PubMed |
Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898.
| Leaf maximum photosynthetic rate and venation are linked by hydraulics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVOgs7s%3D&md5=0f0bcc31dbcd41cfef4c9194f51ea13dCAS | 17556506PubMed |
Buckley TN (2005) The control of stomata by water balance. New Phytologist 168, 275–292.
| The control of stomata by water balance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Smu7bI&md5=00245a1e868c3b96f4891c3b2c5e03d0CAS | 16219068PubMed |
Cochard H, Froux F, Mayr S, Coutand C (2004a) Xylem wall collapse in water-stressed pine needles. Plant Physiology 134, 401–408.
| Xylem wall collapse in water-stressed pine needles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVansrg%3D&md5=bd03e75e841f5ac5767b930dfcdd0f98CAS | 14657404PubMed |
Cochard H, Nardini A, Coll L (2004b) Hydraulic architecture of leaf blades: where is the main resistance? Plant, Cell & Environment 27, 1257–1267.
| Hydraulic architecture of leaf blades: where is the main resistance?Crossref | GoogleScholarGoogle Scholar |
Cochard H, Venisse JS, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S (2007) Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiology 143, 122–133.
| Putative role of aquaporins in variable hydraulic conductance of leaves in response to light.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpt1OgtQ%3D%3D&md5=ca60fb78d4d509f18ded15ad80a7ed30CAS | 17114274PubMed |
Comstock JP (2000) Correction of thermocouple psychrometer readings for the interaction of temperature and actual water potential. Crop Science 40, 709–712.
| Correction of thermocouple psychrometer readings for the interaction of temperature and actual water potential.Crossref | GoogleScholarGoogle Scholar |
DeLucia E, Schlesinger W (1991) Resource-use efficiency and drought tolerance in adjacent Great-Basin and Sierran plants. Ecology 72, 51–58.
| Resource-use efficiency and drought tolerance in adjacent Great-Basin and Sierran plants.Crossref | GoogleScholarGoogle Scholar |
Fernández R, Reynolds J (2000) Potential growth and drought tolerance of eight desert grasses: lack of a trade-off? Oecologia 123, 90–98.
| Potential growth and drought tolerance of eight desert grasses: lack of a trade-off?Crossref | GoogleScholarGoogle Scholar |
Gascó A, Nardini A, Salleo S (2004) Resistance to water flow through leaves of Coffea arabica is dominated by extra-vascular tissues. Functional Plant Biology 31, 1161–1168.
| Resistance to water flow through leaves of Coffea arabica is dominated by extra-vascular tissues.Crossref | GoogleScholarGoogle Scholar |
Girma F, Krieg D (1992) Osmotic adjustment in Sorghum. 2. Relationship to gas-exchange rates. Plant Physiology 99, 583–588.
| Osmotic adjustment in Sorghum. 2. Relationship to gas-exchange rates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XkvVOjtrs%3D&md5=0e4946dc6640053437b28a4bae655515CAS | 16668926PubMed |
Hacke UG, Sperry JS, Wheeler JK, Castro L (2006) Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiology 26, 689–701.
| Scaling of angiosperm xylem structure with safety and efficiency.Crossref | GoogleScholarGoogle Scholar | 16510385PubMed |
Heinen RB, Ye Q, Chaumont F (2009) Role of aquaporins in leaf physiology. Journal of Experimental Botany 60, 2971–2985.
| Role of aquaporins in leaf physiology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsValsb0%3D&md5=71889aaa95e1898406faa35ca70ea226CAS | 19542196PubMed |
Holloway-Phillips MM, Brodribb T (2011a) Minimum hydraulic safety leads to maximum water-use efficiency in a forage grass. Plant, Cell & Environment 34, 302–313.
| Minimum hydraulic safety leads to maximum water-use efficiency in a forage grass.Crossref | GoogleScholarGoogle Scholar |
Holloway-Phillips MM, Brodribb TJ (2011b) Contrasting hydraulic regulation in closely related forage grasses: implications for plant water use. Functional Plant Biology 38, 594–605.
| Contrasting hydraulic regulation in closely related forage grasses: implications for plant water use.Crossref | GoogleScholarGoogle Scholar |
Hubbard RM, Ryan MG, Stiller V, Sperry JS (2001) Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant, Cell & Environment 24, 113–121.
| Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine.Crossref | GoogleScholarGoogle Scholar |
Johnson DM, Mcculloh KA, Woodruff DR, Meinzer FC (2012) Evidence for xylem embolism as a primary factor in dehydration-induced declines in leaf hydraulic conductance. Plant, Cell & Environment 35, 760–769.
| Evidence for xylem embolism as a primary factor in dehydration-induced declines in leaf hydraulic conductance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmtFWls7k%3D&md5=5e6ace0e516b0dc1b973eb80fbad3816CAS |
Jones MM, Turner NC (1978) Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiology 61, 122–126.
| Osmotic adjustment in leaves of sorghum in response to water deficits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXhtVWkuro%3D&md5=0b9272e71ca1e31a2644f35cdc6edb6bCAS | 16660224PubMed |
Kim YX, Steudle E (2007) Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays. Journal of Experimental Botany 58, 4119–4129.
| Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitlymtbs%3D&md5=903fedded05b1bc76b82739a48132d51CAS | 18065766PubMed |
Kodama N, Cousins A, Tu KP, Barbour MM (2011) Spatial variation in photosynthetic CO2 carbon and oxygen isotope discrimination along leaves of the monocot triticale (Triticum × Secale) relates to mesophyll conductance and the Péclet effect. Plant, Cell & Environment 34, 1548–1562.
| Spatial variation in photosynthetic CO2 carbon and oxygen isotope discrimination along leaves of the monocot triticale (Triticum × Secale) relates to mesophyll conductance and the Péclet effect.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1yms7nI&md5=6c6f8a756ea3a15e0370d97809ead878CAS |
Marenco RA, Siebke K, Farquhar GD, Ball MC (2006) Hydraulically based stomatal oscillations and stomatal patchiness in Gossypium hirsutum. Functional Plant Biology 33, 1103–1113.
| Hydraulically based stomatal oscillations and stomatal patchiness in Gossypium hirsutum.Crossref | GoogleScholarGoogle Scholar |
Markesteijn L, Poorter L, Bongers F, Paz H, Sack L (2011) Hydraulics and life history of tropical dry forest tree species: coordination of species’ drought and shade tolerance. New Phytologist 191, 480–495.
| Hydraulics and life history of tropical dry forest tree species: coordination of species’ drought and shade tolerance.Crossref | GoogleScholarGoogle Scholar | 21477008PubMed |
Martre P, Cochard H, Durand JL (2001) Hydraulic architecture and water flow in growing grass tillers (Festuca arundinacea Schreb.). Plant, Cell & Environment 24, 65–76.
| Hydraulic architecture and water flow in growing grass tillers (Festuca arundinacea Schreb.).Crossref | GoogleScholarGoogle Scholar |
McCulloh K, Sperry J, Adler F (2003) Water transport in plants obeys Murray’s law. Nature 421, 939–942.
| Water transport in plants obeys Murray’s law.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsVKgtbw%3D&md5=79966ef6e576430222a94ffa60978736CAS | 12607000PubMed |
McKown AD, Cochard H, Sack L (2010) Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. American Naturalist 175, 447–460.
| Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution.Crossref | GoogleScholarGoogle Scholar | 20178410PubMed |
Meinzer FC (2002) Co-ordination of vapour and liquid phase water transport properties in plants. Plant, Cell & Environment 25, 265–274.
| Co-ordination of vapour and liquid phase water transport properties in plants.Crossref | GoogleScholarGoogle Scholar |
Meinzer F, Grantz D (1990) Stomatal and hydraulic conductance in growing sugarcane – stomatal adjustment to water transport capacity. Plant, Cell & Environment 13, 383–388.
| Stomatal and hydraulic conductance in growing sugarcane – stomatal adjustment to water transport capacity.Crossref | GoogleScholarGoogle Scholar |
Meinzer FC, Goldstein G, Jackson P, Holbrook NM, Gutiérrez MV, Cavelier J (1995) Environmental and physiological regulation of transpiration in tropical forest gap species: the influence of boundary layer and hydraulic properties. Oecologia 101, 514–522.
| Environmental and physiological regulation of transpiration in tropical forest gap species: the influence of boundary layer and hydraulic properties.Crossref | GoogleScholarGoogle Scholar |
Mott KA (2007) Leaf hydraulic conductivity and stomatal responses to humidity in amphistomatous leaves. Plant, Cell & Environment 30, 1444–1449.
| Leaf hydraulic conductivity and stomatal responses to humidity in amphistomatous leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1yhsrbO&md5=adc62657a1d9823faa21e4c0c3225a1dCAS |
Nardini A, Salleo S (2005) Water stress-induced modifications of leaf hydraulic architecture in sunflower: co-ordination with gas exchange. Journal of Experimental Botany 56, 3093–3101.
| Water stress-induced modifications of leaf hydraulic architecture in sunflower: co-ordination with gas exchange.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1GlsbbO&md5=17590d968758405fb1107ab5defa1b22CAS | 16246857PubMed |
Nardini A, Salleo S, Raimondo F (2003) Changes in leaf hydraulic conductance correlate with leaf vein embolism in Cercis siliquastrum L. Trees – Structure and Function 17, 529–534.
| Changes in leaf hydraulic conductance correlate with leaf vein embolism in Cercis siliquastrum L.Crossref | GoogleScholarGoogle Scholar |
Nardini A, Salleo S, Andri S (2005) Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv Margot). Plant, Cell & Environment 28, 750–759.
| Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv Margot).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmvVaku7o%3D&md5=aff3a036aded6805d1a4efe0696542b5CAS |
Nardini A, Pedà G, Rocca NL (2012) Trade-offs between leaf hydraulic capacity and drought vulnerability: morpho-anatomical bases, carbon costs and ecological consequences. New Phytologist 196, 788–798.
| Trade-offs between leaf hydraulic capacity and drought vulnerability: morpho-anatomical bases, carbon costs and ecological consequences.Crossref | GoogleScholarGoogle Scholar | 22978628PubMed |
Ocheltree TW, Nippert JB, Prasad PVV (2012) Changes in stomatal conductance along grass blades reflect changes in leaf structure. Plant, Cell & Environment 35, 1040–1049.
| Changes in stomatal conductance along grass blades reflect changes in leaf structure.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38zosVGhtQ%3D%3D&md5=2b19592a101b9bd8c8ae20b35e4c03ceCAS |
Peak D, Mott KA (2011) A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant, Cell & Environment 34, 162–178.
| A new, vapour-phase mechanism for stomatal responses to humidity and temperature.Crossref | GoogleScholarGoogle Scholar |
R Development Core Team (2011) R: A language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria) Available at http://www.R-project.org/
Ruzin SE (1999) ‘Plant microtechnique and microscopy.’ (Oxford University Press: New York)
Sack L, Frole K (2006) Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 87, 483–491.
| Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees.Crossref | GoogleScholarGoogle Scholar | 16637372PubMed |
Sack L, Holbrook NM (2006) Leaf hydraulics. Annual Review of Plant Biology 57, 361–381.
| Leaf hydraulics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosVKhtrs%3D&md5=3f502b84989933bacc5535c1045bdb19CAS | 16669766PubMed |
Sack L, Melcher P, Zwieniecki MA, Holbrook NM (2002) The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. Journal of Experimental Botany 53, 2177–2184.
| The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xpt1KgtLg%3D&md5=83c383fd6a58330126fe336037d6e958CAS | 12379784PubMed |
Sack L, Streeter C, Holbrook NM (2004) Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology 134, 1824–1833.
| Hydraulic analysis of water flow through leaves of sugar maple and red oak.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjsFKmtrY%3D&md5=b1e95c6fb92ab6c48927d46b96be5d8bCAS | 15064368PubMed |
Sack L, Tyree MT, Holbrook NM (2005) Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytologist 167, 403–413.
| Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees.Crossref | GoogleScholarGoogle Scholar | 15998394PubMed |
Sadok W, Sinclair TR (2010) Transpiration response of ‘slow-wilting’ and commercial soybean (Glycine max (L.) Merr.) genotypes to three aquaporin inhibitors. Journal of Experimental Botany 61, 821–829.
| Transpiration response of ‘slow-wilting’ and commercial soybean (Glycine max (L.) Merr.) genotypes to three aquaporin inhibitors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVSrtbY%3D&md5=d19da63b337bbb76f8d8211c5b0bed9cCAS | 19969533PubMed |
Saliendra N, Sperry J, Comstock J (1995) Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196, 357–366.
| Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXls1Wqs7s%3D&md5=d033b6be43cd09f4a2dacef12c531bf9CAS |
Scoffoni C, Pou A, Aasamaa K, Sack L (2008) The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant, Cell & Environment 31, 1803–1812.
| The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods.Crossref | GoogleScholarGoogle Scholar |
Scoffoni C, Rawls M, McKown A, Cochard H, Sack L (2011) Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiology 156, 832–843.
| Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnvFWrtrk%3D&md5=d56e102ae2e901a986ceb4a54baa6ed3CAS | 21511989PubMed |
Sinclair TR, Zwieniecki MA, Holbrook NM (2008) Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiologia Plantarum 132, 446–451.
| Low leaf hydraulic conductance associated with drought tolerance in soybean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksFWktb4%3D&md5=47d8b0752057897d1ff441203c1fa497CAS | 18333998PubMed |
Sperry JS, Hacke UG (2002) Desert shrub water relations with respect to soil characteristics and plant functional type. Functional Ecology 16, 367–378.
| Desert shrub water relations with respect to soil characteristics and plant functional type.Crossref | GoogleScholarGoogle Scholar |
Sperry J, Donnelly J, Tyree M (1988) A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell & Environment 11, 35–40.
| A method for measuring hydraulic conductivity and embolism in xylem.Crossref | GoogleScholarGoogle Scholar |
Sperry J, Adler F, Campbell G, Comstock J (1998) Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant, Cell & Environment 21, 347–359.
| Limitation of plant water use by rhizosphere and xylem conductance: results from a model.Crossref | GoogleScholarGoogle Scholar |
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49, 419–432.
Trifilò P, Nardini A, Lo Gullo M, Salleo S (2003) Vein cavitation and stomatal behaviour of sunflower (Helianthus annuus) leaves under water limitation. Physiologia Plantarum 119, 409–417.
| Vein cavitation and stomatal behaviour of sunflower (Helianthus annuus) leaves under water limitation.Crossref | GoogleScholarGoogle Scholar |
Tsuda M, Tyree M (2000) Plant hydraulic conductance measured by the high pressure flow meter in crop plants. Journal of Experimental Botany 51, 823–828.
| Plant hydraulic conductance measured by the high pressure flow meter in crop plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtF2jsLY%3D&md5=19854ec3a656993197ecd43e60ce4188CAS | 10938875PubMed |
Tyree MT, Zimmerman MH (2002) Xylem Structure and the Ascent of Sap. (Springer-Verlag: Berlin)
Tyree M, Nardini A, Salleo S, Sack L, El Omari B (2005) The dependence of leaf hydraulic conductance on irradiance during HPFM measurements: any role for stomatal response? Journal of Experimental Botany 56, 737–744.
| The dependence of leaf hydraulic conductance on irradiance during HPFM measurements: any role for stomatal response?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtV2ruro%3D&md5=414a45f8fb0c695279023a14a7d4f2e4CAS | 15582928PubMed |
Wheeler JK, Sperry JS, Hacke UG, Hoang N (2005) Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport. Plant, Cell & Environment 28, 800–812.
| Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport.Crossref | GoogleScholarGoogle Scholar |
Zwieniecki MA, Melcher PJ, Boyce CK, Sack L, Holbrook NM (2002) Hydraulic architecture of leaf venation in Laurus nobilis L. Plant, Cell & Environment 25, 1445–1450.
| Hydraulic architecture of leaf venation in Laurus nobilis L.Crossref | GoogleScholarGoogle Scholar |


 ), SC1205 (
), SC1205 ( ), SC15 (○), TX7078 (⬜).
), SC15 (○), TX7078 (⬜).