Making the best of the worst of times: traits underlying combined shade and drought tolerance of Ruscus aculeatus and Ruscus microglossum (Asparagaceae)
Alexandria Pivovaroff A C , Rasoul Sharifi B , Christine Scoffoni B , Lawren Sack B and Phil Rundel BA Department of Botany and Plant Sciences, University of California, 2150 Batchelor Hall, Riverside, CA 92521, USA.
B Department of Ecology and Evolutionary Biology, University of California, 621 Charles E Young Drive South, Los Angeles, CA 90095-1606, USA.
C Corresponding author. Email: alexandria.pivovaroff@email.ucr.edu
Functional Plant Biology 41(1) 11-24 https://doi.org/10.1071/FP13047
Submitted: 4 March 2013 Accepted: 14 July 2013 Published: 28 August 2013
Abstract
The genus Ruscus (Asparagaceae) consists of evergreen, woody monocot shrubs with modified photosynthetic stems (phylloclades) that occur in dry, shaded woodland areas of the Mediterranean Basin and southern Europe. The combined drought and shade tolerance of Ruscus species challenges the ‘trade-off model’, which suggests that plants can be either drought or shade adapted, but not both. To clarify the potential mechanisms that enable Ruscus species to survive in shaded environments prone to pronounced soil drought, we studied form–function relations based on a detailed trait survey for Ruscus aculeatus L. and Ruscus microglossum Bertol., focusing on gas exchange, hydraulics, morphology, anatomy, and nutrient and isotope composition. We then compared these trait values with published data for other species. R. aculeatus and R. microglossum exhibited numerous traits conferring drought and shade tolerance via reduced demand for resources in general and an ability to survive on stored water. Specific traits include thick phylloclades with low rates of maximum photosynthetic CO2 assimilation, low stomatal conductance to water vapour (gs), low respiration rate, low light compensation point, low shoot hydraulic conductance, low cuticular conductance, and substantial water storage tissue. Ruscus carbon isotope composition values of –33 ‰ were typical of an understory plant, but given the low gs could be associated with internal CO2 recycling. Ruscus appears to be a model for extreme dual adaptation, both physiologically and morphologically, enabling its occupation of shaded sites within drought prone regions across a wide geographical range, including extremely low resource understory sites.
Additional keywords: carbon isotopes, functional morphology, gas exchange, hydraulic conductance, Mediterranean climate, phylloclades, understory.
Introduction
Plant ecological distributions are constrained by several factors including tolerance of environmental conditions such as light and water availability (Sack 2004; Niinemets and Valladares 2006; Hallik et al. 2009; Sterck et al. 2011). According to the ‘trade-off model’ hypothesised by Smith and Huston (1989), a plant’s adaptations can either allow it to tolerate low light or low water availability. However, many plant species have been reported to tolerate sites prone to strong combinations of drought and shade, including Ruscus aculeatus L. (Sack et al. 2003b), which occurs in dry, shaded understory habitats subjected to annual seasonal drought.
Previous studies have shown several species can tolerate combined shade and drought in experiments (Sack 2004; Martínez-Tillería et al. 2012) and in the field (Caspersen 2001; Engelbrecht and Kursar 2003; Niinemets and Valladares 2006), but the physiological mechanisms contributing to this ability have not received detailed study. The ability of Ruscus species to survive very strong combinations of shade and drought in the field and in experiments (Sack et al. 2003b; Sack 2004) makes it a model for such dual adaptation. However, the species have received little detailed study, and previous work has emphasised its adaptation via phenology (de Lillis and Fontanella 1992; Martínez-Pallé and Aronne 1999), high biomass allocation to roots, and its apparent conservative resource use (Sack et al. 2003b).
The objective of this research was to clarify the wide range of potential adaptations of Ruscus that contribute to its remarkable ability to survive and regenerate in shaded sites prone to occasional or seasonal soil drought. To achieve this objective, we studied 57 traits relating to gas exchange, hydraulics, morphology, anatomy, and nutrient and carbon isotope composition in two Ruscus species, R. aculeatus and Ruscus microglossum Bertol. (Fig. 1). We then compared trait values with those hypothesised to confer shade tolerance, drought avoidance or both. Overall, for 21 traits we had a priori hypotheses of a benefit for shade tolerance and for 24 traits we had a priori hypotheses of a benefit for drought avoidance. We then compared the traits for Ruscus with values compiled from the literature for: (1) temperate and tropical broadleaf evergreen species; (2) Mediterranean species; and (3) woody angiosperms in general; in order to put Ruscus trait values in a global context. This approach involved measuring a large number of key aspects of structure and function and, when possible, compiling specific hypotheses for traits potentially involved in the shade and drought tolerance Ruscus species relative to comparator species (Tables 1–5). According to the previous literature on shade and drought tolerance (for example, Givnish 1988; Jones 1992) these suites of traits in Ruscus are expected to directly or indirectly contribute to mechanisms operating across cell types and levels of leaf organisation conferring combined shade and drought adaptation.

|

|

|

|
|

|
Thus, on the general understanding of shade and drought tolerance traits, we hypothesised that Ruscus species would have mechanisms of drought adaptation including traits enabling the delay of tissue dehydration, and traits enabling maintained function even as tissue dehydrates. Such traits include a high water-use efficiency (Wright and Westoby 2003), as well as water storage tissue with high water storage capacitance associated with low bulk leaf modulus of elasticity, high relative water content at turgor loss point, and low cuticular conductance (Sack et al. 2003a; Pasquet-Kok et al. 2010; Ogburn and Edwards 2012). Traits potentially contributing to shade tolerance include low rates of maximum photosynthetic CO2 assimilation per leaf area and per leaf mass, low light compensation point, low maximum rate of carboxylation, low maximum rate of electron transport, and more negative carbon isotope ratios (Walters and Reich 1999). Traits that potentially confer a combined drought and shade tolerance through a general conservative and cost-efficient resource use include thick lamina with thick epidermis, low respiration rates per area and mass, low stomatal conductance, and low shoot hydraulic conductance (Sack et al. 2003b). Given the exceptional biology of these species – their extreme tolerance and their possession of phylloclades – we also qualified additional traits, in particular the detailed anatomical traits such as cell sizes, for which we could not compile hypotheses due to the paucity of comparative data in the published literature. However, such anatomical traits have been argued to be strongly associated with environmental adaptation in principle (Haberlandt 1914), and thus these data for Ruscus are likely to be important as future studies provide comparative data for many species.
Materials and methods
Study species and site
Ruscus (Asparagaceae) is a genus of six species of evergreen sclerophyllous woody shrubs native to western and southern Europe (including north to southern England), Macaronesia, north-west Africa, and south-western Asia ranging east to the Caucasus (de Lillis and Fontanella 1992; Martínez-Pallé and Aronne 1999). Ruscus is thus found in a wide range of temperate forests as well as in Mediterranean-type climates characterised by wet, cool winters and dry, warm summers that result in an annual seasonal period of low water availability, or drought (Matalas 1963; Dracup 1991; Cowling et al. 1996). Ruscus exhibits phylloclades, which are flattened photosynthetic stems that resemble leaves, and are considered intermediate organs that combine stem and leaf features (Fig. 1; Cooney-Sovetts and Sattler 1987). Ruscus aculeatus L. is a typical understory species in forests in the Mediterranean basin (Fig. 1; de Lillis and Fontanella 1992; Martínez-Pallé and Aronne 1999; Sack et al. 2003b). Ruscus microglossum Bertol. is a hybrid produced by crossing species Ruscus hypoglossum L., from the Black Sea region, and Ruscus hypophyllum L., from North Africa (Fig. 1; Thomas 1992; USDA, ARS, National Genetic Resources Program 2009).
Experiments were conducted on R. aculeatus and R. microglossum plants in the Mildred E Mathias Botanical Garden at the University California, Los Angeles, from June to August 2009. Measurements were made in shaded understory sites on at least three individuals of each species. Diurnal light measurements were made on sunny days above the Ruscus plant canopies with a quantum sensor (Li-190S, Li-Cor Biosciences, Lincoln, NE, USA). Individuals received full sunlight averaging ~1500 μmol m–2 s–1 photosynthetically active radiation (PAR) for ~1 h each day, and otherwise experienced PAR of ~50 μmol m–2 s–1 interspersed with sunflecks averaging ~190 μmol m–2 s–1. Plants were irrigated as needed, and all Ruscus individuals were watered before the start of this study to reduce any differences in water availability between individuals.
Phylloclade morphology
We determined leaf morphological traits on recently formed mature phylloclades. Although methods were applied to phylloclades, for convenience we retained the names of methods and traits as applied to leaves (e.g. leaf mass per area). Phylloclade area was measured on excised samples with an area meter (Li-3100, Li-Cor Biosciences). Samples were dried in an oven at >70°C for more than 48 h to determine dry mass and calculate leaf mass per area (LMA) as dry mass divided by area. Phylloclade thickness was measured with electronic digital calipers (Fisher Scientific, Pittsburgh, PA, USA), and density was calculated as mass per area divided by thickness (Witkowski and Lamont 1991).
Phylloclade anatomy
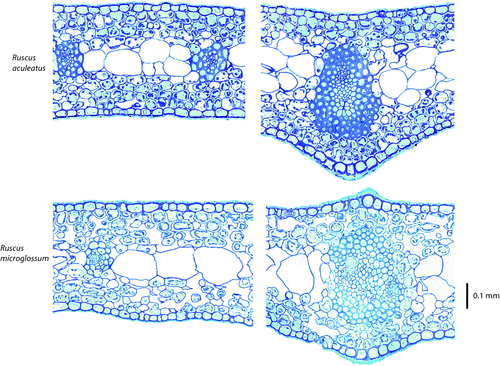
We sampled phylloclades of each species and prepared cross-sections for anatomical measurements. Phylloclades were preserved in formalin acetic acid (37% formaldehyde, glacial acidic acid, 95% ethanol, and deionised water in a 10 : 5 : 50 : 35 mixture). We measured the transverse cross-sectional anatomy using sections cut halfway along the phylloclade length, embedded in LR White (London Resin Co., London, UK), cut 0.5 µm thick using a microtome (Ultracut E, Reichert-Jung Ultracut E, Leica Microsystems, Arcadia, CA, USA), stained with 0.01% toluidine blue in 1% sodium borate, and viewed under the light microscope using a 20–40 × objective (DMRB; Leica Microsystems, Wetzlar, Germany).
We measured tissue thicknesses and cell dimensions in the lamina, and dimensions of vascular bundles and of xylem conduits using Image J software (ver. 1.42 q; National Institutes of Health, Bethesda, MD, USA) on microscope images. We measured thickness for the lamina, cuticle, epidermis, epidermis cell wall, mesophyll, and water storage compartment. We averaged three measurements of each type for each cross-section. The phylloclade tissues were arranged symmetrically, with adaxial and abaxial layers of mesophyll, epidermis and cuticle above and below a central, achlorophyllous water storage tissue (Fig. 2). The per cent air space in each of the adaxial and abaxial mesophyll, and in the water storage compartment was estimated to the nearest 5%, and the total phylloclade airspace was calculated:
|

As indices of cell size, the cross-sectional areas and perimeters were measured for three cells in the epidermis (adaxial and abaxial), the mesophyll (adaxial and abaxial, i.e. above and below the water storage tissue), and the water storage tissue. The area occupied by chloroplasts within a mesophyll cell was measured for three cells (adaxial and abaxial) and the percent cross-sectional chloroplast area was calculated as the ratio of chlorophyll area divided by mesophyll cell area.
We calculated the surface area of mesophyll cells per leaf area (Ames/A) as described by Sack et al. (2013a), a measure of the area available for CO2 uptake for mesophyll cell layers. Given the lack of palisade-form cells, we modelled all mesophyll cells as spheres for these calculations.
We also measured vascular anatomy to quantify traits related to the efficiency of water transport within and outside the xylem. We measured the inter-veinal distance (IVD) and also the minimum distance from edge of bundle sheath to epidermis (Dm) as the hypotenuse between the distance between veins and the distance to the epidermis (Brodribb Feild and Jordan 2007):

We averaged IVD and Dm from three values for each cross-section.
For all anatomical traits, except those relating to the central water storage tissue, measurements were made both adaxial and abaxial halves, and values for the two halves were averaged when not significantly different (at P < 0.05 in paired t-tests), except they were summed for total Ames/A.
To characterise the midrib, for three typical fibrous bundle sheath cells we measured the cross-sectional heights, widths and cell wall thicknesses, calculating mean values for each trait from three measurements per cross-section. To characterise the xylem anatomy and theoretical conductivity of xylem conduits, for a typical conduit within the midrib, an intermediary vein, and a minor vein of each sampled phylloclade, we treated the conduit as an ellipse and determined the major and minor axis diameters. We calculated the theoretical hydraulic conductivity of the xylem conduit using Poiseuille’s equation for ellipses based on conduit dimensions (Lewis and Boose 1995; Cochard Nardini and Coll 2004):

where a and b are the major and minor axes of the ellipse and η is water viscosity at 25°C.
Gas-exchange measurements, responses to light and CO2, and cuticular conductance
In July and August 2009, photosynthetic light response curves and CO2 response curves were measured using a Li-6400 portable photosynthesis system (Li-Cor Biosciences ) with light provided by a red-blue light source (6400–02B no. SI-710; Li-Cor Biosciences ). Gas-exchange measurements were made on at least 1–2 phylloclades per individual.
For light-response curves, phylloclades were acclimated for at least 5 min at 1500 µmol m–2 s–1 PAR, at temperatures of 25−27°C, with RH maintained at ~50%, and CO2 concentration of 400 µmol mol–1. Then, phylloclades were measured for net CO2 assimilation per leaf area at PAR steps of 1600, 1400, 1200, 1000, 800, 600, 500, 400, 300, 200, 100, 50, and 0 µmol m–2 s–1, with 180–240 s stabilisation at each irradiance step. We determined light-saturated photosynthetic rate per area and mass (Aarea and Amass); dark respiration rate per area and mass (Rarea and Rmass), i.e. the negative A at zero PAR; maximum stomatal conductance per area (gs); light compensation point (LCP) as the x-intercept; intrinsic water use efficiency (WUE, Aarea/gs); and the ratio of intercellular to ambient CO2 concentration (Ci/Ca) at 100 µmol m–2 s–1 PAR. We then harvested the phylloclades measured for gas exchange to determine the nitrogen concentration and carbon isotope ratio (see below).
For CO2-response curves, phylloclades were allowed to equilibrate at 400 ppm to induce stomatal opening, and the net CO2 assimilation per leaf area was determined at Ci steps of 400, 300, 200, 100, 50, 400, 400, 500, 600, 800, 1200, 1400, 1600 ppm, with 3–4 min equilibration time at each step. We determined maximum rate of carboxylation and maximum rate of electron transport per leaf area (Vc,max and Jmax) from plots of Ci vs A, corrected to 25°C (Farquhar and von Caemmerer 1980).
Cuticular conductance (i.e. minimum epidermal conductance; gmin sensu Kerstiens 1996) was determined for 3–4 mature phylloclades and 10 cm lengths of stems from each of three individuals of each species. Phylloclade and stem samples were hydrated, and then the cut ends were sealed with wax. Samples were dried for at least 30 min on a laboratory bench, at PAR of <10 µmol photons m–2 s–1 to induce stomatal closure, then samples were weighed for at least eight intervals of 30 min, during which the slope of water loss versus time was highly linear (R2 > 0.995) and therefore taken to represent transpiration after stomata had closed fully. The gmin was calculated as the transpiration rate divided by the mole fraction vapour pressure deficit (VPD, determined from a weather station; HOBO Micro Station with Smart Sensors, Onset, Bourne, MA, USA).
Pressure–volume curve parameters
Pressure–volume curve parameters were determined for mature shoots using the bench-drying method (Koide et al. 2000; Sack et al. 2003a). Shoots were ~10–15 cm long, with about four phylloclades per shoot for R. microglossum and 15–20 phylloclades for R. aculeatus. Shoots were progressively dried on a laboratory bench, and measured at intervals by equilibrating for 10 min in a Whirlpak bag (Whirl-Pak, Nasco, Fort Atkinson, WI, USA) before weighing and measuring for leaf water potential (Ψleaf) with a pressure chamber (Model 1000, Plant Moisture Stress Instruments, Albany, OR, USA). Subsequently, dry mass was determined after more than 48 h in an oven at 70°C. We determined the leaf dry matter content (LDMC), saturated water content (SWC), turgor loss point (Ψtlp), relative water content at turgor loss point (RWCtlp), and osmotic potential at full turgor (πo), relative capacitance at full turgor (Cft; ΔRWC/ΔΨleaf), and relative capacitance at turgor loss point (Ctlp; ΔRWC/ΔΨleaf). We determined the modulus of elasticity (ε) as the linear slope of the line fitted for pressure potential versus relative water content above and including turgor loss point (Sack et al. 2013b).
Shoot hydraulic conductance
We measured the hydraulic conductance of mature shoots (Kshoot) ~10–15 cm long, bearing about four phylloclades per shoot for R. microglossum and 15–20 phylloclades for R. aculeatus, using the evaporative flux method (Sack et al. 2002). Samples were harvested and the ends were re-cut under distilled, degassed water with a fresh razor blade, then hydrated overnight. Shoots were connected to tubing containing distilled, degassed water, running to a graduated cylinder on a balance interfaced to a computer logging data every minute to calculate the flow rate of water from the balance into the sample. Samples were held in place above a fan on wood frames strung with fishing line. Lights were arranged above a plexiglass container of water that acted as a heat trap, producing PAR of >1200 μmol m–2 s–1 at shoot level. When steady-state transpiration was achieved, samples were covered with a plastic bag and removed from the tubing, and the leaf water potential was determined with a pressure chamber (Model 1000, Plant Moisture Stress Instruments). Kshoot was calculated as the steady-state transpirational flow rate (E, mmol m–2 s–1) divided by the water potential driving force (ΔΨleaF = –Ψleaf; MPa), further normalised by total phylloclade area. Kshoot values were standardised to 25°C to correct for the temperature dependence of the viscosity of water (Sack et al. 2003a).
Foliar nitrogen concentration and carbon isotope ratio
To analyse total tissue nitrogen concentration and carbon isotope composition (δ13C), for three replicates from each of three individuals for each species, phylloclade samples were oven-dried at >70°C for more than 48 h, and ground by mortar and pestle. The nitrogen concentration and δ13C were determined at the UC Davis Stable Isotope Facility using an elemental analyser (PDZ Europa ANCA-GSL, Sercon Ltd, Cheshire, UK) interfaced to an isotope ratio mass spectrometer (PDZ Europa 20–20, Sercon Ltd) at the UC Davis Stable Isotope Facility. Final δ13C content values are expressed relative to international standard Vienna Pee Dee belemnite (V-PDB) for carbon:

δ13C of plant tissues can provide a measure of intrinsic WUE (A/gs) at the time that carbon was assimilated, giving long-term water-use efficiency.
Comparative data compilation and trait comparison
To consider Ruscus trait values in a global context, we compiled data from the literature for: (1) temperate and tropical broadleaf evergreen species; (2) Mediterranean species; and (3) woody angiosperms in general. When available, we considered data for plants grown in the shade and for species that are shade tolerant; however, eco-physiological trait data is generally collected for sun leaves or plants, making a comparison solely for shade grown plants not possible. For more commonly studied traits (e.g. Amax), comparative data was taken from studies with large databases and multiple traits (e.g. GLOPNET; Wright et al. 2004). We determined minimum, mean, and maximum values for traits from comparative studies, or the mean and standard error if raw species values were not available. Studies that were included for comparative data are referenced in the captions for Table 1–5 in association with the specific traits we compared. Differences between Ruscus trait values and comparative data from the literature were determined for both R. aculeatus and R. microglossum. Hypotheses were deemed supported for a Ruscus species if the trait value was higher or lower than the mean comparator value in the way predicted.
Results
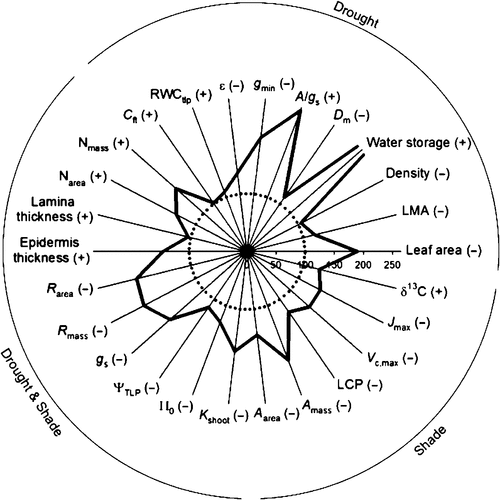
Both Ruscus species studied had leaf morphology consistent with adaptation to shade and drought, relative to comparator species (Tables 1–5). We constructed a radar graph to encapsulate the key traits that would confer adaptation to shade, drought, and the combination, for R. aculeatus, which had the more extreme adaptation of the two Ruscus species considered (Fig. 3). This figure summarises the major results of our study, with values for comparator species appearing as the inner circle and R. aculeatus trait values displayed as the bold, outer line.
|
Gross morphology of phylloclades
Both R. aculeatus and R. microglossum had LMA values lower than comparator species, and R. microglossum had phylloclades larger than the mean leaf area for comparator species. Both Ruscus species had lower bulk tissue density than comparator species, consistent with the water storage in the Ruscus phylloclades. Notably, despite its water storage tissue, the LDMC and SWC values of R. aculeatus were typical of those for comparator species, whereas R. microglossum had a lower LDMC and a higher SWC than for comparator species sets (Table 1).
Anatomy of phylloclades
The Ruscus species possessed numerous anatomical traits consistent with benefits for both shade and drought tolerance (Table 2). The phylloclade cross-sections were symmetrical (Fig. 2), and thus the cross-sectional anatomy of the adaxial and abaxial halves were not different for all traits (paired t-test; P > 0.05) and values were averaged within species. The two species were similar in their substantial leaf thickness and in the thickness of cuticle, epidermis cell walls and water storage compartment (Table 2). The fractions of the lamina occupied by the epidermis, mesophyll and water storage tissues were 15, 52–56, and 29–32% respectively.
Both species had parallel longitudinal veins of three sizes (midrib, intermediate, and small veins). Consistent with drought adaptation, the two species had low Dm, R. aculeatus having the lower value, and R. microglossum had a higher IVD (Table 2). The two species had, on average, the same maximum conduit diameters in their midribs, intermediate veins, and small veins, and the maximum conduit diameter decreased ~35% from the midrib to the small veins. The average theoretical hydraulic conductivity of xylem conduits did not differ between the species, and decreased by up to 86% from the midrib to the minor vein. R. aculeatus had, on average, walls that were 85% thicker in the fibrous bundle sheath (Table 2).
Gas-exchange measurements
Consistent with adaptation to simultaneous shade and drought, the Ruscus species had very low values for Rarea, Rmass, and gs, (Table 3) relative to comparator temperate broadleaf evergreen species (Fig. 3). The Aarea, Amass, LCP, Vc,max and Jmax were also very low relative to comparator species (Fig. 3; Table 3), consistent with shade adaptation. The two Ruscus species had very high values for A/gs (Table 3), consistent with excellent WUE. Consistent with strong drought tolerance via retention of stored water, both species had very low values for leaf and stem gmin, especially relative to comparator vascular plant species (Fig. 3; Table 3).
Hydraulic conductance, pressure volume curve parameters and leaf water storage
Consistent with expectations for combined drought and shade tolerance, both Ruscus species had low Kshoot relative to comparator tropical and temperate woody angiosperms (Fig. 3; Table 4). The pressure–volume curve parameters of Ruscus were consistent with achieving drought tolerance through tissue water storage. Both species had less negative πo and ψtlp than mean values for comparative evergreen woody species (Fig. 3; Table 4). Notably, both species had lower ε values than comparative evergreen woody species, and higher RWCtlp and Cft values (Fig. 3; Table 4), consistent with drought tolerance.
Nitrogen concentration and carbon isotope composition
Both species had high values for phyllyclade Narea and Nmass relative to comparative temperate broadleaf evergreen species, consistent with drought adaptation (Fig. 3). The δ13C values were very negative and typical of values often observed for understory plants (da Silveira et al. 1989), consistent with shade tolerance. Indeed, the δ13C values were notably strongly negative given the high WUE found for these species.
Testing hypotheses for shade and drought tolerance with trait survey data
Overall, we quantified 57 traits for the two Ruscus species, and for 21 traits we had a priori hypotheses for a benefit for shade tolerance and for 24 traits we had a priori hypotheses for a benefit for drought tolerance. Using comparative data, we found that 16 of 21 hypotheses were supported for shade tolerance, and 22 of 24 were supported for drought tolerance. Of the nine hypotheses for traits that would contribute to both shade and drought tolerance simultaneously (i.e. expectations were both for higher or lower values than comparative species), eight were supported by trait data. All these proportions were significantly higher than the 50% support that would have been expected to arise only from chance (P = 0.001–0.058; proportion tests). Notably, in the seven cases when shade tolerance traits would conflict with drought tolerance traits, four indicated a benefit for drought tolerance rather than shade tolerance for both species (Tables 1–5).
Discussion
Both R. aculeatus (Fig. 3) and R. microglossum showed trait values consistent with combined shade and drought tolerance. Numerous traits were consistent with a combined shade and drought tolerance through improving carbon balance, enabling a conservative resource use, i.e. via slow respiration and long-lived parts. Other traits would contribute specifically to drought tolerance via reduced demand for water during active photosynthesis and the ability to survive strong drought after stomatal closure. Notably, many such traits were related to water storage, providing new insights into effective forms of succulence in shaded habitats. This suite of traits would contribute importantly to the ability of Ruscus to occupy shaded sites prone to strong drought across a wide geographical range.
Traits contributing to simultaneous drought and shade tolerance
Ruscus species showed specialisation associated with conservative resource use consistent with tolerance of shade and drought. These specialised traits included thick lamina and component tissues that contribute to long tissue life-spans (shoots last >5 years; Sack et al. 2003b; Wright et al. 2004). Additionally, Ruscus species had very low gas-exchange rates, including low gs, and low Kshoot, which would correspond to a low investment in vascular tissue (Tyree and Zimmermann 1983; Sack et al. 2003b), and low Rarea, Rmass, Aarea and Amass, all representing an ability to maintain photosynthesis and growth with low requirements for light and water.
Traits contributing to drought tolerance
According to Jones et al. (1992), drought tolerance can be achieved through avoidance of plant water deficits, tolerance of plant water deficits or efficiency mechanisms. Ruscus species showed traits associated with drought tolerance either by providing the ability to maintain photosynthesis and growth in drying soil and/or the ability to survive chronic drought, as previously shown experimentally for R. aculeatus (Sack 2004). Traits that would contribute to the ability to maintain gas exchange in drying soil include small leaf size, high WUE and low IVD. High WUE, achieved in part with high Narea, means these Ruscus species can attain positive carbon balance even with extremely low gs. Low IVD and water storage capacitance allow the phylloclades to maintain water supply to the mesophyll and tolerate transiently high evaporation rates, for example, due to sunflecks, without desiccating the leaf (Sack et al. 2003a). Traits contributing to the ability of the phylloclades to survive extended drought included those enabling a low evaporation rate per leaf area once stomata have shut, such as low gmin in leaf and stem, and those related to specialised water storage tissue, linked with low leaf density, low ε, and πo values that were low in magnitude.
Ruscus water storage
The water storage tissue of Ruscus that occupied a third of the leaf thickness, although contributing most directly to drought tolerance, is also consistent with shade tolerance, given its contribution to reduced tissue costs for the phylloclade as a whole. The water storage tissue had thin cell walls, reflected in the low bulk ε, and low solute concentration, contributing to bulk πo values that were low in magnitude.
This water storage tissue would also contribute to both types of drought tolerance – the ability to maintain photosynthesis in drying soil and to survive after stomata have shut during extended drought. The ‘succulence’ of Ruscus phylloclades is distinctive relative to more typical succulent-leafed and succulent-stemmed species, which tend to have high leaf water content and capacitance values (Vendramini et al. 2002; Ogburn and Edwards 2012). In contrast, in Ruscus species, the SWC was low relative to typical leaf succulent species, and for R. aculeatus, Cft fell within the range of typical evergreen leaves. We note that the strong tissue differentiation in Ruscus (i.e. separation of mesophyll cell and water storage in space and their distinctions in anatomy) would contribute to high effectiveness of water storage, even if the bulk tissue overall had low SWC and capacitance. Indeed, across species there tends to be no relationship between the magnitude of SWC or capacitance and the degree of within-leaf tissue differentiation (Ogburn and Edwards 2012). Notably, such differentiation would contribute special advantages for supply of water, whether stomata are open or closed, as the large, thin-walled water storage cells with low solute concentration can yield their water to supply the evaporative load, while the photosynthetic tissues can maintain their volume according to their thicker walls and stronger solute concentration.
Although the capacitance and SWC values were low for Ruscus species, these values would be more substantial if considered relative to water demand. Across several species, Cft has been found to correlate with Kshoot and with gs (Sack et al. 2003a; Blackman et al. 2010), indicating that leaves tend to be built with capacitance to match their maximum flux rates, and thus to buffer the leaf water potential against surges in transpiration. Thus, because Ruscus has low gs when stomata are open and low Kshoot, the capacitance would be expected to supply transpiration transiently during sunflecks or high VPD. Likewise, when stomata close, the capacitance supplies ongoing water loss via cuticular conductance. Given the extremely low gmin of Ruscus, even its moderate Ctlp can enable survival for weeks (Sack et al. 2003b; Sack 2004). Further, at turgor loss, the water content would equal SWC × RWCtlp, and the relatively high RWCtlp would contribute to the high water content once turgor is lost. Thus, the ‘succulence’ of Ruscus is moderate in absolute terms, but combined with its other very strong mechanisms to reduce transpiration when stomata are open or closed, i.e. low gs and gmin, even this moderate capacitance would provide strong functionality.
Water-use efficiency and carbon isotope composition
It was noteworthy that despite extremely high WUE values, phylloclade δ13C values of the Ruscus species were very negative. This presents a strong anomaly worthy of further investigation, as species with high WUE typically have higher δ13C (less negative, i.e. closer to zero). The δ13C can be influenced by a host of processes including source CO2, stored plant carbon, and time-integrated CO2 concentration at the site of carboxylation (Farquhar et al. 1989). For Ruscus, although δ13C values were typical of an understory plant, they did not appear to be driven by internal CO2 concentration because the leaf isotopic values were depleted in 13C, whereas the high WUE determined by gas exchange would likely promote enriched isotopic values. It is more likely that δ13C in this species was determined by source CO2 or stored carbon, or recycling of respired CO2 (da Silveira et al. 1989). Our study individuals of Ruscus were cultivated in a shaded understory, similar to their natural habitat. Previous studies have shown that there can be higher concentrations of respired CO2 in the forest understory than in the canopy, resulting in more negative carbon isotope ratios in understory plant tissue than canopy plant tissue (da Silveira et al. 1989). This is a function of decomposing leaves and litter cover, slow air mixing, as well as plant environmental responses.
A second possible explanation for the δ13C values of Ruscus is related to its growth form and phenology, as Ruscus has extensive rhizomes (Sack et al. 2003b), which act as a carbon store for the plant. Using this recycled carbon during the growth season, when the plant may not be able to meet its carbon requirement by photosynthesis alone because of low maximum rates under light limitation, may also contribute to more negative δ13C values (Vizzini 2003). Notably, Ruscus stems are hollow and thus can also store relatively large amounts of CO2, for use during a growth period when carbon is otherwise limiting, especially given very low gs. Some of this stored carbon might be photosynthetically fixed by the stem (Nilsen and Sharifi 1997). In each of these cases, respired, stored, or recycled CO2 would supply carbon that was previously fixed by Rubisco with more negative δ13C values (–27%o) than air (–8%o).
There was also a dissonance between the δ13C value and Ci/Ca. The fully mature phylloclades which were selected and used for gas-exchange and δ13C measurements were produced during late winter and early spring with mild temperatures (average at midday 19.0–22.0°C) and low atmospheric VPD (average at midday 0.6–1.5 kPa). However, the gas-exchange measurements were taken during summer with high temperature (average at midday 30.0–35.5°C) and high VPD (average at midday 2.5–3.5 kPa). We harvested the phylloclades that we used for gas exchange to determine the carbon isotope ratio. Although the low value of instantaneous Ci/Ca may be caused by high temperature and high atmospheric VPD, the very low value of δ13C may represent the time when the phylloclade’s carbon was assimilated (during mild temperature and low VPD).
Implications for drought and shade tolerance: Ruscus as a model
We found strong support for a large number of hypotheses for trait-based shade and drought tolerance, providing a strong trait basis for combined tolerance. Although the detailed functional trait survey conducted here is relatively novel in its breadth (see also Pasquet-Kok et al. 2010), this is a logical extension of the traditional approach for understanding the basis for plant adaptation to environment, i.e. testing expectations for individual traits established by previous studies of the functional significance of these traits in other species. We acknowledge there is some degree of uncertainty in interpreting a large number of traits simultaneously based on studies of other species. First, the interpretation of the value of traits based on other species may not be in all cases equally valid for Ruscus. Some trait variation may relate to other functions. Further studies, for example, using mutants, would be necessary as conclusive evidence of the value of specific traits in a given species. However, one advantage of testing numerous expectations for each hypothesis is that the key finding will be robust to the removal of some traits from the analysis if those are later found to be inappropriate. Ideally, when a model for estimating plant performance from leaf traits becomes available, one could determine how the specific quantitative combinations of traits presented here scales up to plant shade and drought tolerance.
The shade and drought tolerance of Ruscus, consistent with the suite of traits examined here, is one case demonstrating how plants can avoid a general trade-off between shade and drought tolerance. Further, Ruscus is noteworthy as one of only a few stem photosynthetic plants that occupy a shaded habitat. Historically, it is likely that shade tolerance preceded drought tolerance given this species’ ancestors were species of moist tropical forests (Kim et al. 2010), and thus Ruscus or its ancestor apparently evolved drought tolerance while expanding its range into drier habitat or during past climate change. Considering its unique adaptations and trait values, Ruscus can serve as an excellent model for the basis of combined shade and drought tolerance.
Acknowledgements
We thank the Mildred E Mathias Botanical Garden for access to garden and plants, Andy Truong for assistance with measurements, Howard Griffiths, Louis Santiago, and Cheryl Swift for helpful discussions and comments on the manuscript. This work was supported by National Science Foundation Grant IOB-0546784.
References
Bartlett MK, Scoffoni C, Sack L (2012) The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecology Letters 15, 393–405.| The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis.Crossref | GoogleScholarGoogle Scholar | 22435987PubMed |
Blackman CJ, Brodribb TJ, Jordan GJ (2010) Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytologist 188, 1113–1123.
| Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms.Crossref | GoogleScholarGoogle Scholar | 20738785PubMed |
Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898.
| Leaf maximum photosynthetic rate and venation are linked by hydraulics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVOgs7s%3D&md5=0f0bcc31dbcd41cfef4c9194f51ea13dCAS | 17556506PubMed |
Caspersen JP (2001) Interspecific variation in sapling mortality in relation to growth and soil moisture. Oikos 92, 160–168.
| Interspecific variation in sapling mortality in relation to growth and soil moisture.Crossref | GoogleScholarGoogle Scholar |
Cochard H, Nardini A, Coll L (2004) Hydraulic architecture of leaf blades: where is the main resistance? Plant, Cell & Environment 27, 1257–1267.
| Hydraulic architecture of leaf blades: where is the main resistance?Crossref | GoogleScholarGoogle Scholar |
Cooney-Sovetts C, Sattler R (1987) Phylloclade development in the Asparagaceae: an example of homoeosis. Botanical Journal of the Linnean Society 94, 327–371.
| Phylloclade development in the Asparagaceae: an example of homoeosis.Crossref | GoogleScholarGoogle Scholar |
Cowling R, Rundel P, Lamont BB, Arroyo MK, Arianoutsou M (1996) Plant diversity in Mediterranean-climate regions. Trends in Ecology & Evolution 11, 362–366.
| Plant diversity in Mediterranean-climate regions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itFGjtg%3D%3D&md5=04037125a761a1804f0f1ddecdab8d78CAS |
da Silveira L, Sternberg L, Mulkey SS, Wright SJ (1989) Ecological interpretation of leaf carbon isotope ratios: influence of respired carbon dioxide. Ecology 70, 1317–1324.
| Ecological interpretation of leaf carbon isotope ratios: influence of respired carbon dioxide.Crossref | GoogleScholarGoogle Scholar |
de Lillis M, Fontanella A (1992) Comparative phenology and growth in different species of the Mediterranean maquis of central Italy. Plant Ecology 99-100, 83–96.
| Comparative phenology and growth in different species of the Mediterranean maquis of central Italy.Crossref | GoogleScholarGoogle Scholar |
Dracup JA (1991) Drought monitoring. Stochastic Hydrology and Hydraulics 5, 261–266.
| Drought monitoring.Crossref | GoogleScholarGoogle Scholar |
Engelbrecht BMJ, Kursar TA (2003) Comparative drought-resistance of seedlings of 28 species of co-occurring tropical woody plants. Oecologia 136, 383–393.
| Comparative drought-resistance of seedlings of 28 species of co-occurring tropical woody plants.Crossref | GoogleScholarGoogle Scholar |
Farquhar GD, von Caemmerer S (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90.
| A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXksVWrt7w%3D&md5=b0417117f3598df013ab17a3da1f67a8CAS |
Farquhar GD, Ehleringer JR, Hubick K (1989) Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40, 503–537.
| Carbon isotope discrimination and photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXktlKmu70%3D&md5=47e2104f39b18af9dc9829574923d822CAS |
Givnish TJ (1988) Adaptation to sun and shade: a whole-plant perspective. Australian Journal of Plant Physiology 15, 63–92.
| Adaptation to sun and shade: a whole-plant perspective.Crossref | GoogleScholarGoogle Scholar |
Gulias J, Flexas J, Mus M, Cifre J, Lefi E, Medrano H (2003) Relationship between maximum leaf photosynthesis, nitrogen content and specific leaf area in Balearic endemic and non-endemic Mediterranean species. Annals of Botany 92, 215–222.
| Relationship between maximum leaf photosynthesis, nitrogen content and specific leaf area in Balearic endemic and non-endemic Mediterranean species.Crossref | GoogleScholarGoogle Scholar | 12805082PubMed |
Haberlandt G (1914) ‘Physiological plant anatomy.’ (MacMillan: London)
Hallik L, Niinemets U, Wright J (2009) Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora? New Phytologist 184, 257–274.
| Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1Kitb%2FN&md5=8406fab9b4187c016e4544b159a84bf9CAS | 19674334PubMed |
Jones HG (1992) ‘Plants and microclimate: a quantitative approach to environmental plant physiology.’ (Cambridge University Press: Cambridge)
Kerstiens G (1996) Cuticular water permeability and its physiological significance. Journal of Experimental Botany 47, 1813–1832.
| Cuticular water permeability and its physiological significance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXotFGitw%3D%3D&md5=e3728c3e46f7068c8e03a35eac518a2bCAS |
Kim JH, Kim DK, Forest F, Fay MF, Chase MW (2010) Molecular phylogenetics of Ruscaceae sensu lato and related families (Asparagales) based on plastid and nuclear DNA sequences. Annals of Botany 106, 775–790.
| Molecular phylogenetics of Ruscaceae sensu lato and related families (Asparagales) based on plastid and nuclear DNA sequences.Crossref | GoogleScholarGoogle Scholar | 20929900PubMed |
Koide RT, Robichaux RH, Morse SR, Smith CM (2000) Plant water status, hydraulic resistance and capacitance. In ‘Plant physiological ecology: field methods and instrumentation’. (Eds R Pearcy, JR Ehleringer, H Mooney, P Rundel) pp. 161–183. (Kluwer: Dordrecht, The Netherlands)
Körner C, Farquhar GD, Wong SC (1991) Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecologia 88, 30–40.
| Carbon isotope discrimination by plants follows latitudinal and altitudinal trends.Crossref | GoogleScholarGoogle Scholar |
Lewis AM, Boose ER (1995) Estimating volume flow rates through xylem conduits. American Journal of Botany 82, 1112–1116.
| Estimating volume flow rates through xylem conduits.Crossref | GoogleScholarGoogle Scholar |
Martínez-Pallé E, Aronne G (1999) Flower development and reproductive continuity in Mediterranean Ruscus aculeatus L. (Liliaceae). Protoplasma 208, 58–64.
| Flower development and reproductive continuity in Mediterranean Ruscus aculeatus L. (Liliaceae).Crossref | GoogleScholarGoogle Scholar |
Martínez-Tillería K, Loayza AP, Sandquist DR, Squeo FA (2012) No evidence of a trade-off between drought and shade tolerance in seedlings of six coastal desert shrub species in north-central Chile. Journal of Vegetation Science 23, 1051–1061.
| No evidence of a trade-off between drought and shade tolerance in seedlings of six coastal desert shrub species in north-central Chile.Crossref | GoogleScholarGoogle Scholar |
Matalas NC (1963) Probability distribution of low flows. USGS Professional Papers 434-A, US Government Printing Office, Washington DC.
Niinemets U (1999) Research review: components of leaf dry mass per area – thickness and density – alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytologist 144, 35–47.
| Research review: components of leaf dry mass per area – thickness and density – alter leaf photosynthetic capacity in reverse directions in woody plants.Crossref | GoogleScholarGoogle Scholar |
Niinemets U, Valladares F (2006) Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecological Monographs 76, 521–547.
| Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs.Crossref | GoogleScholarGoogle Scholar |
Nilsen ET, Sharifi MR (1997) Carbon isotope composition of legumes with photosynthetic stems from Mediterranean and desert habitats. American Journal of Botany 84, 1707–1713.
| Carbon isotope composition of legumes with photosynthetic stems from Mediterranean and desert habitats.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MnjvVCktQ%3D%3D&md5=8bc18fd504d2667542e363f0734e2472CAS |
Ogburn RM, Edwards EJ (2012) Quantifying succulence: a rapid, physiologically meaningful metric of plant water storage. Plant, Cell & Environment 35, 1533–1542.
| Quantifying succulence: a rapid, physiologically meaningful metric of plant water storage.Crossref | GoogleScholarGoogle Scholar |
Pasquet-Kok J, Creese C, Sack L (2010) Turning over a new ‘leaf’: multiple functional significances of leaves versus phyllodes in Hawaiian Acacia koa. Plant, Cell & Environment 33, 2084–2100.
| Turning over a new ‘leaf’: multiple functional significances of leaves versus phyllodes in Hawaiian Acacia koa.Crossref | GoogleScholarGoogle Scholar |
Sack L (2004) Responses of temperate woody seedlings to shade and drought: do trade-offs limit potential niche differentiation? Oikos 107, 110–127.
| Responses of temperate woody seedlings to shade and drought: do trade-offs limit potential niche differentiation?Crossref | GoogleScholarGoogle Scholar |
Sack L, Frole K (2006) Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 87, 483–491.
| Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees.Crossref | GoogleScholarGoogle Scholar | 16637372PubMed |
Sack L, Holbrook NM (2006) Leaf hydraulics. Annual Review of Plant Biology 57, 361–381.
| Leaf hydraulics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosVKhtrs%3D&md5=3f502b84989933bacc5535c1045bdb19CAS | 16669766PubMed |
Sack L, Melcher PJ, Zwieniecki MA, Holbrook NM (2002) The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. Journal of Experimental Botany 53, 2177–2184.
| The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xpt1KgtLg%3D&md5=83c383fd6a58330126fe336037d6e958CAS | 12379784PubMed |
Sack L, Cowan P, Jaikumar N, Holbrook NM (2003a) The “hydrology” of leaves: co‐ordination of structure and function in temperate woody species. Plant, Cell & Environment 26, 1343–1356.
| The “hydrology” of leaves: co‐ordination of structure and function in temperate woody species.Crossref | GoogleScholarGoogle Scholar |
Sack L, Grubb P, Maronon T (2003b) The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain. Plant Ecology 168, 139–163.
| The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain.Crossref | GoogleScholarGoogle Scholar |
Sack L, Scoffoni C, McKown AD, Frole K, Rawls M, Havran JC, Tran H, Tran T (2012) Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nature Communications 3, 837
| Developmentally based scaling of leaf venation architecture explains global ecological patterns.Crossref | GoogleScholarGoogle Scholar | 22588299PubMed |
Sack L, Chatelet D, Scoffoni C, PrometheusWiki contributors (2013a) “Estimating the mesophyll surface area per leaf area from leaf cell and tissue dimensions measured from transverse cross-sections,” PrometheusWiki. Available at http://www.publish.csiro.au/prometheuswiki/tiki-pagehistory.php?page=Estimating the mesophyll surface area per leaf area from leaf cell and tissue dimensions measured from transverse cross-sections&preview=16 [accessed June 18, 2013]
Sack L, Pasquet-Kok J, PrometheusWiki contributors (2013b) “Leaf pressure-volume curve parameters,” PrometheusWiki. Available at http://prometheuswiki.publish.csiro.au/tiki-index.php?page=Leaf+pressure-volume+curve+parameters.
Scoffoni C, Pou A, Aasamaa K, Sack L (2008) The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant, Cell & Environment 31, 1803–1812.
| The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods.Crossref | GoogleScholarGoogle Scholar |
Smith T, Huston M (1989) A theory of the spatial and temporal dynamics of plant communities. Vegetatio 83, 49–69.
| A theory of the spatial and temporal dynamics of plant communities.Crossref | GoogleScholarGoogle Scholar |
Sterck F, Markesteijn L, Schieving F, Poorter L (2011) Functional traits determine trade-offs and niches in a tropical forest community. Proceedings of the National Academy of Sciences of the United States of America 108, 20 627–20 632.
| Functional traits determine trade-offs and niches in a tropical forest community.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xkt1Krsw%3D%3D&md5=68aa8bb6cf42ea54e53286e17d0fd972CAS |
Thomas GS (1992) ‘Ornamental shrubs: climbers and bamboos.’ (John Murray Publishers: Great Britain)
Tyree MT, Zimmermann MH (1983) ‘Xylem structure and the ascent of sap.’ (Springer-Verlag: Berlin)
USDA, ARS, National Genetic Resources Program (2009) Germplasm Resources Information Network (GRIN) online database. National Germplasm Resources Laboratory, Beltsville, Maryland. Available athttp://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?410577 [Accessed 20 July 2009]
Vendramini F, Díaz S, Gurvich DE, Wilson PJ, Thompson K, Hodgson JG (2002) Leaf traits as indicators of resource‐use strategy in floras with succulent species. New Phytologist 154, 147–157.
| Leaf traits as indicators of resource‐use strategy in floras with succulent species.Crossref | GoogleScholarGoogle Scholar |
Vile D (2005) Specific leaf area and dry matter content estimate thickness in laminar leaves. Annals of Botany 96, 1129–1136.
| Specific leaf area and dry matter content estimate thickness in laminar leaves.Crossref | GoogleScholarGoogle Scholar | 16159941PubMed |
Vizzini S (2003) δ13C and δ15N variability in Posidonia oceanica associated with seasonality and plant fraction. Aquatic Botany 76, 195–202.
| δ13C and δ15N variability in Posidonia oceanica associated with seasonality and plant fraction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvVCmsbg%3D&md5=2e385eb8329570255027a5792f42e3ceCAS |
Walters M, Reich PB (1999) Research review: low-light carbon balance and shade tolerance in the seedlings of woody plants: do winter deciduous and broad-leaved evergreen species differ? New Phytologist 143, 143–154.
| Research review: low-light carbon balance and shade tolerance in the seedlings of woody plants: do winter deciduous and broad-leaved evergreen species differ?Crossref | GoogleScholarGoogle Scholar |
Witkowski E, Lamont BB (1991) Leaf specific mass confounds leaf density and thickness. Oecologia 88, 486–493.
Wright IJ, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Functional Ecology 17, 10–19.
| Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species.Crossref | GoogleScholarGoogle Scholar |
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428, 821–827.
| The worldwide leaf economics spectrum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjt1Crt74%3D&md5=5c596807f20b8b7b19a8c1c726dde6ccCAS | 15103368PubMed |
Wullschleger SD (1993) Biochemical limitations to carbon assimilation in C3 plants – a retrospective analysis of the A/C i curves from 109 species. Journal of Experimental Botany 44, 907–920.
| Biochemical limitations to carbon assimilation in C3 plants – a retrospective analysis of the A/C i curves from 109 species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXkvFWnurk%3D&md5=8277cf55c29caa6456c2558fe8d55140CAS |


