Pot size matters: a meta-analysis of the effects of rooting volume on plant growth
Hendrik Poorter A C , Jonas Bühler A , Dagmar van Dusschoten A , José Climent B and Johannes A. Postma AA IBG-2 Plant Sciences, Forschungszentrum Jülich, D-52425, Germany.
B INIA, Forest Research Centre, Department of Forest Ecology and Genetics, Avda A Coruña Km 7.5., 28040 Madrid, Spain.
C Corresponding author. Email: h.poorter@fz-juelich.de
Functional Plant Biology 39(11) 839-850 https://doi.org/10.1071/FP12049
Submitted: 16 February 2012 Accepted: 11 May 2012 Published: 15 June 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
The majority of experiments in plant biology use plants grown in some kind of container or pot. We conducted a meta-analysis on 65 studies that analysed the effect of pot size on growth and underlying variables. On average, a doubling of the pot size increased biomass production by 43%. Further analysis of pot size effects on the underlying components of growth suggests that reduced growth in smaller pots is caused mainly by a reduction in photosynthesis per unit leaf area, rather than by changes in leaf morphology or biomass allocation. The appropriate pot size will logically depend on the size of the plants growing in them. Based on various lines of evidence we suggest that an appropriate pot size is one in which the plant biomass does not exceed 1 g L–1. In current research practice ~65% of the experiments exceed that threshold. We suggest that researchers need to carefully consider the pot size in their experiments, as small pots may change experimental results and defy the purpose of the experiment.
Additional keywords: container volume, experimental setup, meta-analysis, pot size, plant growth, rooting volume.
Introduction
A large number of studies in plant biology focus on gene expression, physiology or biomass production of individually-grown plants. To this end, experiments are often conducted on plants grown in some kind of container, from here on referred to as ‘pots’. Pot size has received special attention in forestry (Carlson and Endean 1976) and horticulture (Kharkina et al. 1999), where commercial companies can profit from choosing the smallest pot volume that still delivers a product with an appropriate quality. Occasionally, the issue of pot size has received attention in other fields of plant biology. Arp (1991), for example, emphasised that pot size might be an important issue in experiments that considered the effect of elevated CO2 on plants; and recently, a discussion in the field of ecophysiology has emerged, where studies on the recognition of roots of neighbours are thought to be confounded by pot size (Hess and De Kroon 2007).
Apart from the fields mentioned above, pot size seems to have received little consideration in the scientific literature and is regulary not reported in the materials and methods section of publications. Nonetheless, it is an important issue. In most laboratories there is large demand for growth chamber facilities and the use of small pots generally implies more experiments or increased replication. Space is probably less of an issue in most glasshouses. However, the plant biology community currently makes a great effort to develop automated systems for plant phenotyping (Granier et al. 2006; Nagel et al. 2012). These high-throughput systems allow for the handling of many plants, which automatically implies that spatial demands on growth facilities will increase. The use of small pots has the additional advantage that it does not exceed the capabilities of the transport robots to move the weight of pot plus plant.
The use of small pots for research purposes may also have disadvantages, which are more related to biological constraints. A small container implies a small quantity of soil or other substrate and thereby, almost invariably, a reduction in the availability of water and nutrients to the plant. In addition to reduced resource availability, pots generally impede root growth. Many species easily produce roots of more than 1 m in length (Jackson et al. 1996), even at a relative young age (Drew 1975; Fusseder 1987), thereby exceeding the dimensions of most containers used. Large plants in small pots may have a large fraction of roots ‘pot-bound’, with all kind of secondary consequences (Herold and McNeil 1979). In this paper we explore the consequences of the choice of pot size for plants studied for experimental purposes. We first analysed in a quantitative way to what extent pot size affects plant growth in the studies that explicitly considered this factor. We did so by a meta-analysis of 65 studies reported in the literature. Second, we reported on some of the morphological and physiological components that might explain the observed growth patterns. Third, we analysed whether there is a threshold in the plant size : pot size relationship above which growth is affected and compared the data for the current pot-size experiments with a more general database on plant research and to plants grown in fields. Based on these comparisons we suggest what might be an appropriate pot size for a given experiment.
1. Effect of pot size on plant biomass
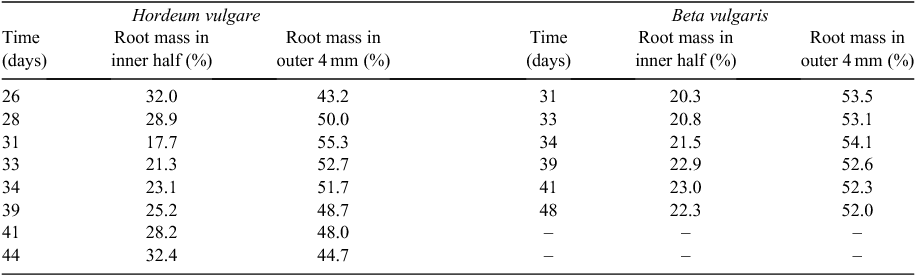
To analyse the effect of rooting volume on plant growth, we screened the literature of the last 100 years and arrived at a total of 65 publications where this factor was studied in pots and ~10 where rooting volume was constrained in hydroponically-grown plants (see Appendix 1). For this analysis we will focus mainly on the results for the pot-grown plants. A description of the methodological approach is given in Appendix 2. The effect of pot size has been studied in a wide range of pot volumes, with values ranging from 5 mL for a herbaceous greenhouse crop (Bar-Tal and Pressman 1996), to 1700 L for trees growing over a period of several years (Hsu et al. 1996). The range of pot sizes used within the various experiments was also large, varying by a factor 2 at least and a factor 35 at most. A clear example in our compilation is the experiment by Endean and Carlson (1975), who followed the growth of Pinus contorta Douglas over time. In the very beginning of the experiment, plants grew presumably well in all pot sizes, but after 4 weeks of growth biomass was already reduced in the smallest pot volume (Fig. 1a), and by the last harvest, none of the pots seemed large enough to ensure unrestricted growth.
|
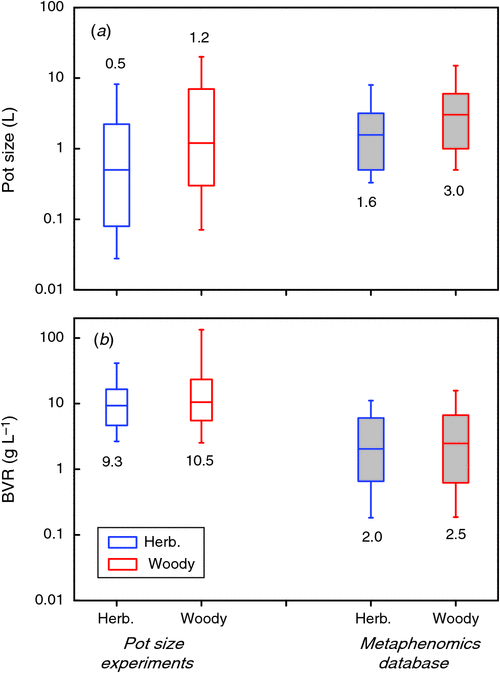
In the full compiled data set the picture is similar: in almost all of the experiments considered plants increase in weight with every larger pot that is used (Fig. 1b). Most experiments fall between the dotted lines, which indicate a fixed ratio of biomass to pot volume of 2 and 100 g L–1, respectively, for the lower and higher line. Only a few experiments with trees in large containers exceed the 100 g L–1. As all experiments start with small seedlings, plants move over time from the bottom part of the graph upwards (cf. Fig. 1a). Clearly, the experiments at the upper right end of Fig. 1b are also the ones that were planned to last for the longest time, 2 years in case of the experiment with the largest pots (Hsu et al. 1996), 5 years in case of the experiment with the highest ratio (Bar-Yosef et al. 1988).
Theoretically, dose–response curves should level off at higher pot sizes, as in Fig. 1a. However, this is not the case for many of the studies plotted in Fig. 1b, which mostly show linear responses. We discuss this further in section 4. To scale all data to an equal change in pot size, we calculated for each species in each of the experiments the average slope of the log-transformed mass versus pot size relationship and derived from those values an easy to understand expression that indicates the percentage increase in biomass for a 100% increase in pot size (Appendix 3). On average, plants increased 43% in mass for every doubling in pot size (Fig. 2, P < 0.001), with no significant differences in response between herbaceous and woody species. These response values are substantial and imply that plants are likely to be over 3 times larger in 2 L pots than in pots of 0.2 L. Given these differences, it is clear that pot size should be given careful consideration during the planning phase of an experiment.
|
2. Effect on components of the carbon-budget
What is the cause of the growth retardation in smaller pot sizes? As clearly shown by Endean and Carlson (1975), the effect of pot size on biomass gradually increases throughout (the later part of) the experimental period. The rate by which biomass of individual plants is accumulated is proportional to the size of the plant and conveniently described by the relative growth rate (RGR, the rate of increase in biomass per unit biomass present; Evans 1972). The differences in RGR of plants growing in different pot sizes are always smaller than the differences in biomass at the end of the experiment. This implies that the physiological and morphological factors that underlie the variation in biomass will also be affected to a smaller extent than biomass itself (Poorter et al. 2012a). We analysed growth in terms of the plant’s carbon economy, using a top-down approach where the RGR is factorised into three components:

according to Evans (1972). Here, SLA denotes specific leaf area (leaf area per unit leaf dry mass; m2 kg–1), LMF the relative allocation of biomass to leaves (leaf mass fraction, g leaf g–1 plant) and ULR is a parameter that indicates the growth rate per unit leaf area (unit leaf rate, g m–2 day–1). ULR is basically the net result of carbon gain through photosynthesis, corrected for the rate of respiration in the whole plant and the C-content of the newly added biomass. ULR and photosynthesis per unit leaf area are often strongly positively correlated (Poorter 2002).
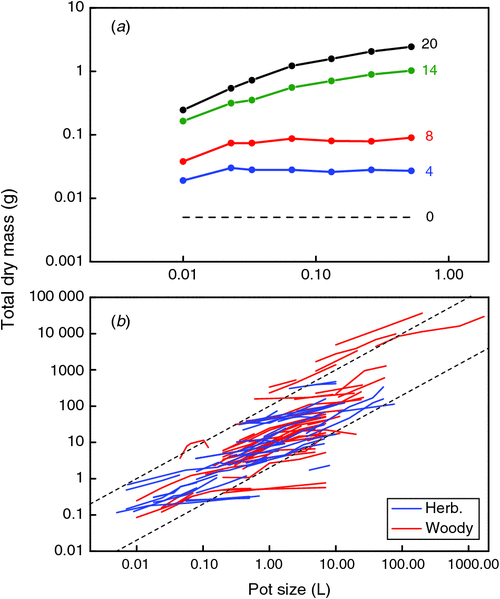
A characteristic of RGR is that an absolute difference in RGR between treatments causes a relative difference in biomass over time (Poorter and Navas 2003). However, in the case where we want to understand the reason for the difference in growth and only have fragmented information, it is more amenable to analyse the proportional differences in RGR relative to that of the three growth parameters that underlie RGR (Eqn 1). Only a few experiments report data on these underlying components, but the information we could gather points into the following direction (see Fig. 2 and Fig. S1, available as Supplementary Material to this paper): a doubling in pot size increases RGR by ~5%. Consequently, each of the growth parameters at the right-hand side of Eqn 1 may change by a very small proportion, or one of them by a somewhat larger proportion in the range of 5%. At the final harvest, SLA increased somewhat in some experiments and decreased in others. We could quantitatively compare the various experiments by calculating the percentage increase in SLA with a 100% increase in pot size and found that taken over all experiments, this variable did not deviate significantly from zero (Fig. 2). Most, but not all experiments with root restriction in hydroponics confirm this response (e.g. Carmi et al. 1983; Kharkina et al. 1999; but see Tschaplinski and Blake 1985).
Allocation patterns are more frequently reported, generally as the biomass ratio between shoot and root. We prefer a classification in at least three plant organs (Poorter et al. 2012b) and therefore use LMF, SMF and RMF, which are the fractions of total vegetative biomass invested to leaves, stems and roots respectively. The relatively scarce information indicates that LMF is not affected (Fig. 2a), whereas SMF increased slightly but non-significantly (0.05 < P < 0.10) with pot size. More information is present on RMF (or the root : shoot ratio). Reviews are not equivocal in their evaluation. NeSmith and Duval (1998) conclude that RMF often does not change with pot size. In contrast, Hess and De Kroon (2007) expect RMF to increase in larger pots. We found that over ~80 species × experiment combinations, RMF decreased on average by a small but significant extent (4% with a doubling in pot size, P < 0.05) We find no clear differences between woody and herbaceous plants in this respect (data not shown).
With two of the variables of Eqn 1 unaffected, we might expect that the observed differences in growth rates between plants grown in different pot sizes are caused largely by a change in net photosynthesis and hence we would expect a stimulation of ~4% in this variable. Some experiments report a more than 30% higher rate of photosynthesis with a 100% increase in pot size (Robbins and Pharr 1988; Ronchi et al. 2006; Fig. S1g). This is far more than the expected 4%. As pot size stress builds up gradually over time, results may depend on the actual timing of the measurements during the experiment. Moreover, obtained rates will also depend on the specific leaf measured and the time of the day that the measurement was taken. The growth parameter ULR is generally closely linked to the rate of photosynthesis and provides an estimate that is integrated over the whole plant and the whole growth period under consideration (Poorter 2002). A 2-fold difference is not reported in the literature (Fig. S1f), but the pot-size effects on ULR are significantly greater than zero and larger than for SLA and LMF (Fig. 2). The median increase in photosynthesis and ULR with a doubling in pot size is quite comparable to the median increase in RGR. Hence, we conclude that with the limited and fragmentary evidence yet available, net photosynthesis is likely to be the process that is strongest affected by pot size and may explain best the observed pot size effect on biomass (Fig. 1b). Additional support comes from experiments where photosynthesis recovered quickly after plants were repotted in larger rooting volumes (Herold and McNeil 1979).
3. What mechanism could explain a reduced photosynthesis in smaller pots?
Several factors could explain the reduced rate of photosynthesis – and thereby growth – in smaller pots. A first possible explanation is that containers of smaller dimensions can be placed at a higher density, with less light available for each shoot and hence, a lower rate of photosynthesis. Although this could be the case in some of the compiled experiments, strong pot size effects on biomass and photosynthesis are generally also observed when density is specifically controlled for (e.g. Endean and Carlson 1975; Robbins and Pharr 1988; NeSmith et al. 1992; Climent et al. 2011).
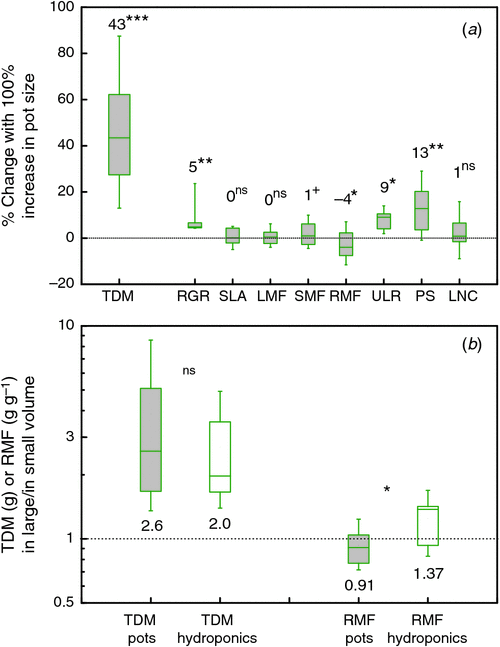
In the type of experiments that we included in our meta-analysis, a smaller pot size will inadvertently decrease the total nutrient content in the pot. Low nitrogen and phosphorus availability are known to decrease photosynthesis (e.g., Sinclair and Horie 1989; Lynch et al. 1991) and growth and increase the root mass fraction (Poorter et al. 2012b). Thus, lower resource supply could form a plausible explanation. We calculated the response of leaf nitrogen concentrations to changes in pot size, expecting to see an increase if nutrient availability would explain the pot size effect. On average, there was a slight, but non-significant increase in leaf nitrogen concentrations with pot size (Fig. 2), suggesting that this factor cannot completely explain the observed differences in photosynthesis or growth. Similar results were found for phosphorus (Krizek et al. 1985). This conclusion is to a certain extent supported by observations on hydroponically-grown plants, which have decreased photosynthesis and growth (Fig. 2b) when the root volume was restricted, despite a continuous high supply of nutrients. However, unlike plants grown in pots, hydroponically-grown plants do not show an increased RMF when restricted (Fig. 2b). As increased RMF is a good indicator for nutrient stress, we presume that nutrient limitation in small pots is still a factor, although we cannot exclude possible allometric effects which could explain a larger RMF in smaller plants as well (Poorter et al. 2012b).
Water is the other commodity that may be in short supply. Small pots could negatively impact the water status of plants as they have a reduced total water holding capacity and will, therefore, dry out more quickly (Tschaplinski and Blake 1985) and at severe stress levels increase RMF (Poorter et al. 2012b). Ray and Sinclair (1998) demonstrated with their drought experiment that soil in small pots dries out faster and thereby caused more severe drought stress in plants. However, pot size does not necessarily affect stomatal conductance or leaf water potential (Ronchi et al. 2006) and – as for nutrients – with plants growing in hydroponics there is still a clear effect of root confinement, even though water availability is not restricted.
Besides resource availability, the temperature of the rooting volume could be affected by pot size (de Vries 1980). Pots can intercept a substantial amount of solar radiation especially in experimental gardens and glasshouses, which may increase the soil temperature at the edge and eventually in the middle of the pot if no precautions are made (Martini et al. 1991; Xu et al. 2001). Small pots have greater surface areas relative to their volume and thereby heat up more quickly. Townend and Dickinson (1995) measured 5°C higher day temperatures in 0.19 L pots compared with 1.9 L pots. Keever et al. (1986) suggest that the greater temperature fluctuations in small pots may explain the reduced growth of the plants. High temperatures in the pot may have several direct (respiration, root growth) and indirect (through increased microbial activity) effects on plant growth. Pot temperatures are rarely reported so it is difficult to evaluate how often temperature differences between pots contribute to reduced growth. However, given that growth reductions also have been observed in hydroponically-grown plants suggests that temperature differences alone cannot explain the observed pot effects either.
If neither nutrient or water availability nor higher temperatures can (fully) explain the pot size effects on photosynthesis and growth, it could be that root confinement per se may cause growth retardation, with reduced photosynthesis as a consequence. Root growth is known to respond directly to impedance. Impeded roots stay shorter whereas the initiation and growth of side branches increases (Bengough and Mullins 1991; Falik et al. 2005). Furthermore, Young et al. (1997) showed that within 10 min of increasing the impedance to root growth, leaf expansion rate is reduced. This suggests that some kind of signal may regulate shoot growth when a large proportion of the roots are impeded. The actual signal for such a response remains as yet unknown. Possibly a reduced sink strength of the root system could cause a direct negative feedback on photosynthesis (Paul and Pellny 2003). Alternatively, a specific root–shoot signal is involved (Jackson 1993).
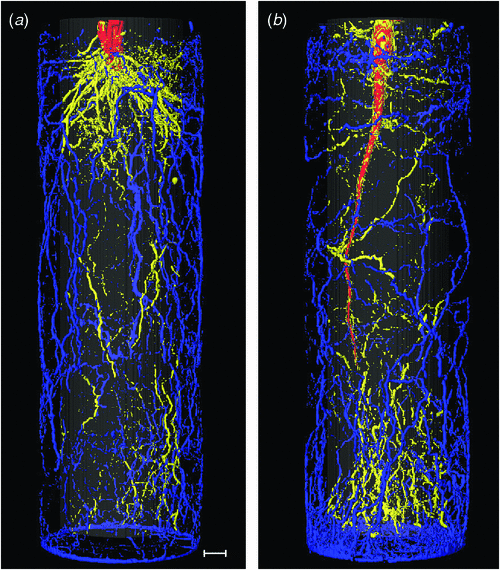
A crucial point in the evaluation of the lastly discussed option is knowledge on the actual distribution of roots within the pots. Although the vertical root distribution is relatively easily measured (Price et al. 2002; Suriyagoda et al. 2010), analysis of the horizontal distributions is more complicated. Using non-destructive magnetic resonance imaging (MRI), we followed the root development of Hordeum vulgare L. and Beta vulgaris L. plants over time in three dimensional space. Representative nuclear magnetic resonance (NMR) images of root systems at the end of the experiment are shown in Fig. 3. We calculated the percentage of roots that was located in the inner half of the soil volume, furthest away from wall and bottom and the percentage of roots present in the outer 4 mm of the pot. Only 20–25% of the root biomass was in the inner part of the pot (Table 1), whereas ~50% was found in the outer 4 mm (20% of the total volume). The proportional distribution remained remarkably constant over time. Hence, if these observations have wider validity, we conclude that a relatively large fraction of roots is close to the edge of the pot, where unfavourable environmental conditions, for example, large temperature fluctuations and impedance of the pot wall may negatively impact growth.
|
4. When does pot size limitation starts?
In sections 1 and 2 we considered for each experiment the overall effect on plant growth, morphology and physiology when pot size was doubled. However, it is to be expected that a plant of a given size will be constrained more in a small than in a large pot. That is, young plants are initially not affected by pot size, but as plants grow older, the pot size effect becomes more pronounced, even in medium-sized pot volumes (Fig. 1a). When experiments last for sufficiently long time, even the largest pot size might not be large enough for unrestricted growth, i.e. the saturating part of the curve extends beyond the largest pots used. For the experimental data this implies that the relationship becomes close to linear again. In fact, many of the curves in Fig. 1b show a linear relationship. For experiments where only two pot sizes were used, it is impossible to deduce whether the response of the plants is indeed linear or not. But even in many of the other experiments in Fig. 1b no clear saturation is shown. What is the reason for that?
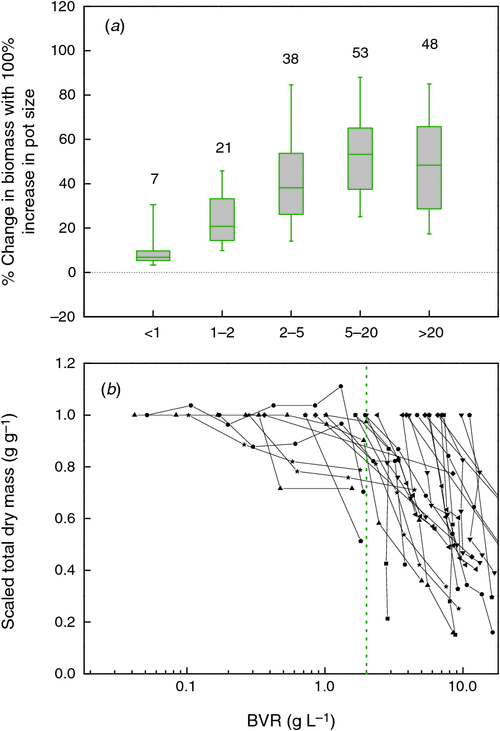
One objective way to relate plant and pot size across experiments is to calculate the plant biomass that is present at a given volume of rooting space. This variable, for which we use BVR as an acronym (total plant biomass : rooting volume ratio; g L–1), has, to our knowledge, been used only by Kerstiens and Hawes (1994). BVR values vary widely and ranged in our database from as low as 0.01 in work by Climent et al. (2008) to over 300 g L–1 in work reported by Biran and Eliassaf (1980). The median value in the pot size experiments is around 9.5 for experiments both with herbaceous and woody species (Fig. 4b). We tested whether the BVR could explain the form of the dose–response curves in the 65 experiments shown in Fig. 1b, by calculating for each point what the slope of the dose–response curves was, as well as the BVR. In order to be consistent with Fig. 2, we derived the percentage increase in biomass with pot size doubling for these data as well. We found that very few experiments had BVR values lower than 2 g L–1 (Fig. 4b). Hence, for this part of the analysis we included not only data of the last harvest, but also data of earlier harvests where available. This may imply that not all data points in the analysis are formally independent, but it increases the power of our analysis in this crucial range. We binned all values in five BVR ranges and show the resulting distribution in Fig. 5a. Estimation of slopes is always more challenging than determining the absolute values per point and this may be one of the reasons that there is considerable variation within each category. In the category with a BVR between 1 and 2 g L–1 the effect of pot size is clearly noticeable. Pot size effects are saturating when BVR values exceed the 2 g L–1 (P < 0.01).
|
|
Another way to obtain a greater insight into the relationship between plant biomass and pot size is to express the biomass of plants grown at various pot sizes relative to the biomass at the largest pot size and plot these values against the BVR (Fig. 5b). Experiments where pot sizes are limiting growth throughout the full range of pot sizes are characterised by lines that decline linearly with BVR. However, as long as biomass is not affected, the line will remain around 1 and only drop at greater BVR values when pot size starts to reduce growth. For the few experiments where this was the case, we could show that this inflection point occurred somewhere between 0.2 and 2 g L–1 (Fig. 5b). Thus, from both the full sets of experiments compiled, as for the more detailed analyses over time, we derive that pot size effects are particularly strong when BVR values are greater than 2 g L–1.
5. How do these BVR values relate to other experiments and the field?
As mentioned in section 1, the range of pot sizes used in this compilation is large. The median value is around 0.9 L (Fig. 4a). How does that compare to common practice in ecophysiological experiments? This will partly depend on the species studied. Arabidopsis, for example, is generally grown in much smaller pots (with an interquartile range of 0.08–0.21 L) than Zea mays L. (1.8–5.0 L). An overall impression of used pot sizes can be obtained from the metaphenomics database described by Poorter et al. (2010), where the response of ~900 different species to 12 different environmental factors is compiled for a total of ~800 experiments from the literature. The median pot size used in that compilation of experiments turns out to be ~2–3 times larger than those in the pot size studies (Fig. 4a), whereas the median BVR value is ~4-fold lower (Fig. 4b). Hence, we conclude that most studies on pot sizes have focussed on relatively small pots and have grown plants to larger sizes than is common in ecophysiological experiments. In contrast, experiments in plant biology generally use relatively larger pots and harvest plants at younger stages, when the BVR value is still below 8 g L–1.
Most experiments with pots are conducted to eventually understand how (agro-)ecosystems function. It may therefore be relevant to consider what normal ‘BVR’ values are in the field. Maximum dry matter yield of major crops varies between 1000–3000 g m–2 (Unkovich et al. 2010; assuming 20% biomass in roots). If we assume a rooting depth of 1 m, this would correspond to BVR values in the range of 1–3 g L–1. Given that at least half of the DM production takes place during grain filling, we can expect that during the vegetative state, the BVR value will not exceed 1.5 g L–1. Similar calculations for natural ecosystems are more difficult as large variation exists in root depth and the standing biomass. Given a rooting depth of 0.35 m (Nagel et al. 2012) and a density of 500 plants per m2, a field of Arabidopsis thaliana Heynh. plants of 0.1 g dry mass, would have a BVR of 0.15 g L–1. Although we realise that plants in the field experience conditions that are very different from those where plants are grown singly in pots in controlled conditions, we conclude from these rough estimates that BVR values around 1 are of the same order of magnitude as those of vegetative plants in the field.
6. Does pot size affect experimental conclusions?
Up to now we have considered the effect of pot size per se. Most researchers are also interested in whether the outcome of their experiments is affected by the choice of the pot size. Arp (1991) was one of the first to draw attention to the fact that pot size might restrict the response to elevated CO2. This would limit the possibilities to draw conclusions from experiments that have been conducted in this field.
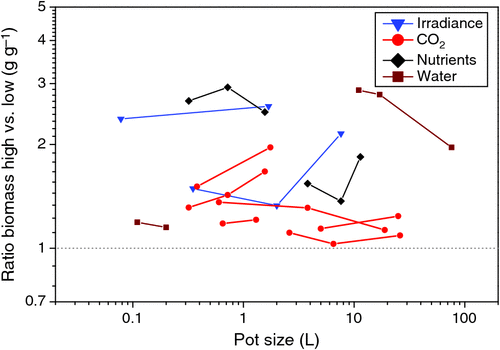
The analysis by Arp (1991) was a compilation of different studies that worked with different pot sizes. Kerstiens and Hawes (1994), however, published a meta-analysis of the results of a range of studies with trees in which they show that the biomass responses to elevated CO2 did not correlate with pot size, or even decreased with a BVR over 18 g L–1. This suggests that small pot sizes do not reduce responses to elevated CO2 universally. However, in both meta-analyses the evidence could only be circumstantial, as pot size was not an experimental factor in the compilations. Here we analysed experiments where pot size was specifically included in the experimental design, not only for interaction with CO2 but also for nutrients, water and irradiance (Fig. 6). Given that nutrient and water availability already increase when pot size is increased, we would expect less additional effect on plant growth if more nutrients or water were supplied. However, we would expect increasingly stronger growth responses with larger pot size when light or CO2 would be increased, simply because of the higher demand for nutrients and water in larger plants. Although some of the experiments do indeed follow the expected trend, results are not equivocal. As results depend on the variability in at least four different harvests, the number of experiments is likely too small to draw any strong conclusion.
|
Besides possible interactions with abiotic factors, interactions with biotic factors have been shown. For example, vesicular arbuscular mycorhizae (VAM) infection rates, which would normally increase with reduced nutrient availability, are reduced in small pots (Bååth and Hayman 1984; Koide 1991). As a consequence, VAM colonisation is less beneficial for nutrient uptake and results in smaller growth increases when small pots are used (Kucey and Janzen 1987; Koide 1991). Similarly, Baldwin (1988) showed that pot-bound Nicotiana plants do not respond to leaf damage, whereas repotted plants do. Thus, although we cannot draw a firm conclusion here we suggest that the use of small pots with crowded roots carries a substantial risk of influencing the experimental results.
7. Other considerations
This analysis focussed on the effect of pot volume on plant growth. However, choosing a pot for an experiment not only includes choosing the right volume, but also the right shape. Although shape is less important than volume (McConnaughay et al. 1993), shallow and deep-rooting species may respond differently to the actual diameter and height of the pots, at equal pot volume (von Felten and Schmid 2008). Pot height is also an important factor in determining the free-draining water content of pots and thereby the water potential as well as the oxygen availability in the pots (Passioura 2006).
An alternative to standard plastic pots are containers that have ribbed inner sides and small air holes. Such containers promote self-pruning of roots close to the holes, which avoids root spiralling and promotes development of lateral roots (Rune 2003). Not only shape, but also the material (Bunt and Kulwiec 1970) and the colour of the pot (Markham et al. 2011) may affect plant growth, mainly through their effect on soil and root temperature. For a broader discussion on the use of pots for growing plants in the context of experimental setup see Poorter et al. (2012a).
Conclusions
A meta-analysis of the effects of pot size on growth shows that on average a doubling of the pot size results in 43% more biomass. In most cases reduced growth in small pots will be caused by a reduction in net photosynthesis. It is the plant mass per unit rooting volume that is relevant rather than pot size per se. Large plant mass per pot volume not only reduces growth of plants but also carries the risk of influencing the relative differences between treatments. We conclude that it is important for researchers to minimise such effects by choosing pots that are large enough for their plants, even at later stages of growth. Our advice is to avoid plant biomass to pot volume ratio’s larger than 2 g L–1 and preferably work with plant and pot sizes where this ratio is <1.
Acknowledgements
Damian Barrett, Phil Grime, Joyce Latimer, Liesje Mommer and Ronald Pierik kindly provided (additional) unpublished data for this analysis. Christian Heinemann enlightened us with the mathematical aspects and comments of Liesje Mommer, Thijs Pons, Marc Faget as well as two anonymous reviewers on a previous version of the ms are greatly appreciated as well.
References
Arp W (1991) Effects of source–sink relations on photosynthetic acclimation to elevated CO2. Plant, Cell & Environment 14, 869–875.| Effects of source–sink relations on photosynthetic acclimation to elevated CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhsVyns7g%3D&md5=f0af8ff7293134af1f1bcce4f80e7d9eCAS |
Bååth E, Hayman DS (1984) Effect of soil volume and plant density on mycorrhizal infection and growth response. Plant and Soil 77, 373–376.
| Effect of soil volume and plant density on mycorrhizal infection and growth response.Crossref | GoogleScholarGoogle Scholar |
Baldwin IT (1988) Damage-induced alkaloids in tobacco: pot-bound plants are not inducible. Journal of Chemical Ecology 14, 1113–1120.
| Damage-induced alkaloids in tobacco: pot-bound plants are not inducible.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXktlGgu70%3D&md5=ff98994d67c6c184447d6df3d3e41109CAS |
Bar-Tal A, Pressman E (1996) Root restriction and potassium and calcium solution concentrations affect dry-matter production, cation uptake, and blossom-end rot in greenhouse tomato. Journal of the American Society for Horticultural Science 121, 649–655.
Bar-Yosef B, Schwartz S, Markovich T, Lucas B, Assaf R (1988) Effect of root volume and nitrate solution concentration on growth, fruit yield, and temporal N and water uptake by apple trees. Plant and Soil 107, 49–56.
| Effect of root volume and nitrate solution concentration on growth, fruit yield, and temporal N and water uptake by apple trees.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXktlOhs7Y%3D&md5=33403f93ac2e51cb03c45728a3d52f6cCAS |
Barrett DJ, Gifford RM (1995) Photosynthetic acclimation to elevated CO2 in relation to biomass allocation in cotton. Journal of Biogeography 22, 331–339.
| Photosynthetic acclimation to elevated CO2 in relation to biomass allocation in cotton.Crossref | GoogleScholarGoogle Scholar |
Bengough A, Mullins C (1991) Penetrometer resistance, root penetration resistance and root elongation rate in two sandy loam soils. Plant and Soil 131, 59–66.
Bilderback T (1985) Growth response of Leyland cypress to media, N application and container size after 1 and 2 growing seasons. Journal of Environmental Horticulture 3, 132–135.
Biran I, Eliassaf A (1980) The effect of container size and aeration conditions on growth of roots and canopy of woody plants. Scientia Horticulturae 12, 385–394.
| The effect of container size and aeration conditions on growth of roots and canopy of woody plants.Crossref | GoogleScholarGoogle Scholar |
Bunt AC, Kulwiec ZJ (1970) The effect of container porosity on root environment and plant growth. I. Temperature. Plant and Soil 32, 65–80.
| The effect of container porosity on root environment and plant growth. I. Temperature.Crossref | GoogleScholarGoogle Scholar |
Carlson LW, Endean F (1976) The effect of rooting volume and container configuration on the early growth of white spruce seedlings. Canadian Journal of Forest Research 6, 221–224.
| The effect of rooting volume and container configuration on the early growth of white spruce seedlings.Crossref | GoogleScholarGoogle Scholar |
Carmi A, Hesketh JD, Enos WT, Peters DB (1983) Interrelationships between shoot growth and photosynthesis, as affected by root growth restriction. Photosynthetica 17, 240–245.
Centritto M (2000) Source-sink relations affect growth but not the allocation pattern of birch (Betula pendula Roth) seedlings under elevated [CO2]. Plant Biosystems 134, 31–37.
| Source-sink relations affect growth but not the allocation pattern of birch (Betula pendula Roth) seedlings under elevated [CO2].Crossref | GoogleScholarGoogle Scholar |
Climent J, Alonso J, Gil L (2008) Short note: root restriction hindered early allometric differentiation between seedlings of two provenances of Canary Island pine. Silvae Genetica 57, 4–5.
Climent J, Chambel MR, Pardos M, Lario F, Villar-Salvador P (2011) Biomass allocation and foliage heteroblasty in hard pine species respond differentially to reduction in rooting volume. European Journal of Forest Research 130, 841–850.
| Biomass allocation and foliage heteroblasty in hard pine species respond differentially to reduction in rooting volume.Crossref | GoogleScholarGoogle Scholar |
de Vries MPC (1980) How reliable are results of pot experiments? Communications in Soil Science and Plant Analysis 11, 895–902.
| How reliable are results of pot experiments?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXlvVCrs78%3D&md5=fc918d9866b13426e5dfa97d13be86f7CAS |
Drew MC (1975) Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist 75, 479–490.
| Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XlslOkug%3D%3D&md5=eba115adaa1c1301390ecbc844ecf85fCAS |
Endean F, Carlson L (1975) The effect of rooting volume on the early growth of lodgepole pine seedlings. Canadian Journal of Forest Research 5, 55–60.
| The effect of rooting volume on the early growth of lodgepole pine seedlings.Crossref | GoogleScholarGoogle Scholar |
Evans GC (1972) ‘The quantitative analysis of plant growth.’ (Blackwell Scientific Publications: Oxford)
Falik O, Reides P, Gersani M, Novoplansky A (2005) Root navigation by self inhibition. Plant, Cell & Environment 28, 562–569.
| Root navigation by self inhibition.Crossref | GoogleScholarGoogle Scholar |
Fusseder A (1987) The longevity and activity of the primary root of maize. Plant and Soil 101, 257–265.
| The longevity and activity of the primary root of maize.Crossref | GoogleScholarGoogle Scholar |
Granier C, Aguirrezabal L, Chenu K, Cookson SJ, Dauzat M, Hamard P, Thioux JJ, Rolland G, Bouchier-Combaud S, Lebaudy A, Muller B, Simonneau T, Tardieu F (2006) PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytologist 169, 623–635.
| PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit.Crossref | GoogleScholarGoogle Scholar |
Herold A, McNeil PH (1979) Restoration of photosynthesis in pot-bound tobacco plants. Journal of Experimental Botany 30, 1187–1194.
| Restoration of photosynthesis in pot-bound tobacco plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXptFWmsA%3D%3D&md5=0eb6738c69732f0d95e81bdb168d7605CAS |
Hess L, De Kroon H (2007) Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination. Journal of Ecology 95, 241–251.
| Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination.Crossref | GoogleScholarGoogle Scholar |
Houle G, Babeux P (1998) The effects of collection date, IBA, plant gender, nutrient availability, and rooting volume on adventitious root and lateral shoot formation by Salix planifolia stem cuttings from the Ungava Bay area (Quebec, Canada). Canadian Journal of Botany 76, 1687–1692.
Hsu Y, Tseng M, Lin C (1996) Container volume affects growth and development of wax-apple. HortScience 31, 1139–1142.
Ismail AM, Hall AE, Bray EA (1994) Drought and pot size effects on transpiration efficiency and carbon isotope discrimination of cowpea. Australian Journal of Plant Physiology 21, 23–35.
| Drought and pot size effects on transpiration efficiency and carbon isotope discrimination of cowpea.Crossref | GoogleScholarGoogle Scholar |
Jackson MB (1993) Are plant hormones involved in root to shoot communication. Advances in Botanical Research 19, 103–187.
| Are plant hormones involved in root to shoot communication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXislCjtw%3D%3D&md5=056cb365d8f3f8e88bca612600c1fe60CAS |
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108, 389–411.
| A global analysis of root distributions for terrestrial biomes.Crossref | GoogleScholarGoogle Scholar |
Jahnke S, Menzel I, Van Dusschoten D, Roeb GW, Bühler J, Minwuyelet S, Blümler P, Temperton VM, Hombach T, Streun M, Beer S, Khodaverdi M, Ziemons K, Coenen HH, Schurr U (2009) Combined MRI–PET dissects dynamic changes in plant structures and functions. The Plant Journal 59, 634–644.
| Combined MRI–PET dissects dynamic changes in plant structures and functions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFChtrrK&md5=0790bd18cc5392c909273062b6c0461eCAS |
Keever GJ, Cobb GS, McDaniel R (1986) Effects of container size, root pruning, and fertilization on growth of seedling pecans. Journal of Environmental Horticulture 4, 11–13.
Kerstiens G, Hawes C (1994) Response of growth and carbon allocation to elevated CO2 in young cherry (Prunus avium L.) saplings in relation to root environment. New Phytologist 128, 607–614.
| Response of growth and carbon allocation to elevated CO2 in young cherry (Prunus avium L.) saplings in relation to root environment.Crossref | GoogleScholarGoogle Scholar |
Kharkina T, Ottosen CO, Rosenqvist E (1999) Effects of root restriction on the growth and physiology of cucumber plants. Physiologia Plantarum 105, 434–441.
| Effects of root restriction on the growth and physiology of cucumber plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjsFSisL0%3D&md5=e3dcfc4ad3cf4851b2a97d0a1bf53b9dCAS |
Koide RT (1991) Density-dependent response to mycorrhizal infection in Abutilon theophrasti Medic. Oecologia 85, 389–395.
| Density-dependent response to mycorrhizal infection in Abutilon theophrasti Medic.Crossref | GoogleScholarGoogle Scholar |
Krizek DT, Carmi A, Mirecki RM, Snyder FW, Bunce JA (1985) Comparative effects of soil moisture stress and restricted root zone volume on morphogenetic and physiological responses of soybean (Glycine max (L.) Merr.). Journal of Experimental Botany 36, 25–38.
| Comparative effects of soil moisture stress and restricted root zone volume on morphogenetic and physiological responses of soybean (Glycine max (L.) Merr.).Crossref | GoogleScholarGoogle Scholar |
Kucey RMN, Janzen H (1987) Effects of VAM and reduced nutrient availability on growth and phosphorus and micronutrient uptake of wheat and field beans under greenhouse conditions. Plant and Soil 104, 71–78.
| Effects of VAM and reduced nutrient availability on growth and phosphorus and micronutrient uptake of wheat and field beans under greenhouse conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXjs1Oltw%3D%3D&md5=bb8c98f132620e9152b130c5d7adc457CAS |
Liu A, Latimer JG (1995) Water relations and abscisic acid levels of watermelon as affected by rooting volume restriction. Journal of Experimental Botany 46, 1011–1015.
| Water relations and abscisic acid levels of watermelon as affected by rooting volume restriction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXnslWltbc%3D&md5=375b596c09aed73814217c2f594c9a49CAS |
Lynch JP, Lauchli A, Epstein E (1991) Vegetative growth of the common bean in response to phosphorus nutrition. Crop Science 31, 380–387.
| Vegetative growth of the common bean in response to phosphorus nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXisVKgurc%3D&md5=a5030ab977d939de430efdbfcc429e36CAS |
Markham JW, Bremer DJ, Boyer CR, Schroeder KR (2011) Effect of container color on substrate temperatures and growth of red maple and redbud. HortScience 46, 721–726.
Martini CA, Ingram DL, Nell TA (1991) Growth and photosynthesis of Magnolia grandiflora ‘St Mary’ in response to constant and increased container volume. Journal of the American Society for Horticultural Science 116, 439–445.
McConnaughay KDM, Berntson G, Bazzaz F (1993) Limitations to CO2-induced growth enhancement in pot studies. Oecologia 94, 550–557.
| Limitations to CO2-induced growth enhancement in pot studies.Crossref | GoogleScholarGoogle Scholar |
McGinley M, Smith C, Elliott P, Higgins J (1990) Morphological constraints on seed mass in lodgepole pine. Functional Ecology 4, 183–192.
| Morphological constraints on seed mass in lodgepole pine.Crossref | GoogleScholarGoogle Scholar |
Nagel KA, Putz A, Gilmer F, Heinz K, Fischbach A, Pfeifer J, Faget M, Bloßfeld S, Ernst M, Dimaki C, Kastenholz B, Kleinert AK, Galinski A, Scharr H, Fiorani F, Schurr U (2012) GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Functional Plant Biology 39, 891–904.
| GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons.Crossref | GoogleScholarGoogle Scholar |
NeSmith DS (1993) Summer squash response to root restriction under different light regimes 1. Journal of Plant Nutrition 16, 765–780.
| Summer squash response to root restriction under different light regimes 1.Crossref | GoogleScholarGoogle Scholar |
NeSmith DS, Duval JR (1998) The effect of container size. HortTechnology 8, 495–498.
NeSmith DS, Bridges DC, Barbour JC (1992) Bell pepper responses to root restriction. Journal of Plant Nutrition 15, 2763–2776.
| Bell pepper responses to root restriction.Crossref | GoogleScholarGoogle Scholar |
Nobel PS, Cui M, Miller PM, Luo Y (1994) Influences of soil volume and an elevated CO2 level on growth and CO2 exchange for the crassulacean acid metabolism plant Opuntia ficus-indica. Physiologia Plantarum 90, 173–180.
| Influences of soil volume and an elevated CO2 level on growth and CO2 exchange for the crassulacean acid metabolism plant Opuntia ficus-indica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhvVOgsb8%3D&md5=558bc137eaa0a3175e9c0b4b7d4be73bCAS |
Passioura JB (2006) The peril of pot experiments. Functional Plant Biology 33, 1075–1079.
| The peril of pot experiments.Crossref | GoogleScholarGoogle Scholar |
Paul MJ, Pellny TK (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. Journal of Experimental Botany 54, 539–547.
| Carbon metabolite feedback regulation of leaf photosynthesis and development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsFKgtbY%3D&md5=516a54d108362e80a2d4603cfef2d7a7CAS |
Poorter H (2002) Plant growth and carbon economy. In ‘Encyclopedia of life sciences’. (Nature Publishing Group: London) Available at: http://www.els.net
Poorter H, Navas ML (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytologist 157, 175–198.
| Plant growth and competition at elevated CO2: on winners, losers and functional groups.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Niinemets Ü, Walter A, Fiorani F, Schurr U (2010) A method to construct dose–response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data. Journal of Experimental Botany 61, 2043–2055.
| A method to construct dose–response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVGgu7c%3D&md5=c8e451e3dffd51a92dab1637095adbd7CAS |
Poorter H, Fiorani F, Stitt M, Schurr U, Finck A, Gibon Y, Usadel B, Munns R, Atkin OK, Tardieu F, Pons TL (2012a) The art of growing plants for experimental purposes; a practical guide for the plant biologist. Functional Plant Biology 39, 839–850.
| The art of growing plants for experimental purposes; a practical guide for the plant biologist.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012b) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. Tansley Review. New Phytologist 193, 30–50.
| Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. Tansley Review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitVKgtr0%3D&md5=3f829a7e95af99a7567d4f3d4d14246eCAS |
Price AH, Steele KA, Gorham J, Bridges JM, Moore BJ, Evans JL, Richardson P, Jones RGW (2002) Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes. I. Root distribution, water use and plant water status. Field Crops Research 76, 11–24.
| Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes. I. Root distribution, water use and plant water status.Crossref | GoogleScholarGoogle Scholar |
R Development Core Team (2011) R: A language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria) Available at: http://www.R-project.org/
Ray JD, Sinclair TR (1998) The effect of pot size on growth and transpiration of maize and soybean during water deficit stress. Journal of Experimental Botany 49, 1381–1386.
Robbins NS, Pharr DM (1988) Effect of restricted root growth on carbohydrate metabolism and whole plant growth of Cucumis sativus L. Plant Physiology 87, 409–413.
Ronchi CP, DaMatta FM, Batista KD, Moraes GABK, Loureiro ME, Ducatti C (2006) Growth and photosynthetic down-regulation in Coffea arabica in response to restricted root volume. Functional Plant Biology 33, 1013–1023.
| Growth and photosynthetic down-regulation in Coffea arabica in response to restricted root volume.Crossref | GoogleScholarGoogle Scholar |
Rune G (2003) Slits in container wall improve root structure and stem straightness of outplanted scots pine seedlings. Silva Fennica 37, 333–342.
Sinclair TR, Horie T (1989) Leaf nitrogen, photosynthesis, and crop radiation use efficiency: a review. Crop Science 29, 90–98.
| Leaf nitrogen, photosynthesis, and crop radiation use efficiency: a review.Crossref | GoogleScholarGoogle Scholar |
Suriyagoda LDB, Ryan MH, Renton M, Lambers H (2010) Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus- and moisture-limited environments. Annals of Botany 105, 755–767.
| Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus- and moisture-limited environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlsFCjtrg%3D&md5=2dc7cd019a72eb432079eeaa09ed6b01CAS |
Thomas RB, Strain BR (1991) Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiology 96, 627–634.
| Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXksVKgsrc%3D&md5=bb8daef78d8b433b52e982a06936908eCAS |
Townend J, Dickinson AL (1995) A comparison of rooting environments in containers of different sizes. Plant and Soil 175, 139–146.
| A comparison of rooting environments in containers of different sizes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXot1Kgsr4%3D&md5=3bae079d495ecea924c8bab08dc798a7CAS |
Tschaplinski TJ, Blake TJ (1985) Effects of root restriction on growth correlations, water relations and senescence of alder seedlings. Physiologia Plantarum 64, 167–176.
| Effects of root restriction on growth correlations, water relations and senescence of alder seedlings.Crossref | GoogleScholarGoogle Scholar |
Unkovich M, Baldock J, Forbes M (2010) Variability in harvest index of grain crops and potential significance for carbon accounting: examples from Australian agriculture. Advances in Agronomy 105, 173–219.
| Variability in harvest index of grain crops and potential significance for carbon accounting: examples from Australian agriculture.Crossref | GoogleScholarGoogle Scholar |
von Felten S, Schmid B (2008) Complementarity among species in horizontal versus vertical rooting space. Journal of Plant Ecology 1, 33–41.
| Complementarity among species in horizontal versus vertical rooting space.Crossref | GoogleScholarGoogle Scholar |
Will R, Teskey R (1997) Effect of elevated carbon dioxide concentration and root restriction on net photosynthesis, water relations and foliar carbohydrate status of loblolly pine seedlings. Tree Physiology 17, 655–661.
| Effect of elevated carbon dioxide concentration and root restriction on net photosynthesis, water relations and foliar carbohydrate status of loblolly pine seedlings.Crossref | GoogleScholarGoogle Scholar |
Xu G, Wolf S, Kafkafi U (2001) Interactive effect of nutrient concentration and container volume on flowering, fruiting, and nutrient uptake of sweet pepper. Journal of Plant Nutrition 24, 479–501.
| Interactive effect of nutrient concentration and container volume on flowering, fruiting, and nutrient uptake of sweet pepper.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktlOlsL8%3D&md5=93d384c54d43daba8f79b66c10b2d5e6CAS |
Young IM, Montagu K, Conroy J, Bengough AG (1997) Mechanical impedance of root growth directly reduces leaf elongation rates of cereals. New Phytologist 135, 613–619.
| Mechanical impedance of root growth directly reduces leaf elongation rates of cereals.Crossref | GoogleScholarGoogle Scholar |
Appendix 1. List of references used for the meta-analysis
A. Pot studies
Cris and Stout (1929) Agric. Exp. Stat. MSC, No 6.; Stevenson (1967) Can. J. Soil Sci. 47, 163–174; Endean and Carlson (1975) Can. J. For. Res. 5, 55–60; Hocking and Mitchell (1975) Can. J. For. Res. 5, 440–451; Carlson and Endean (1976) Can. J. For. Res. 6, 221–224; Herold and McNeil (1979) J. Exp. Bot. 30, 1187–1194; Biran and Eliassaf (1980) Sci. Hortic. 12, 385–394; Peterson et al. (1984) Agron. J. 76, 861–863; Bilderback (1985) J. Env. Hortic. 3, 132–135; Krizek et al. (1985) J. Exp. Bot. 36, 25–38; Carmi (1986) Field Crops Res. 13, 25–32; Hanson et al. (1987) HortSci. 22, 1293–1295; Kucey and Janzen (1987) Plant Soil 104, 71–78; Ruff et al. (1987) J. Amer. Soc. Hort. Sci. 112, 763–769; Tilt et al. (1987) J. Amer. Soc. Hort. Sci. 112, 981–984; Bar-Yosef et al. (1988) Plant and Soil 107, 49–56; Robbins and Pharr (1988) Plant Physiol. 87, 409–413; Bar-Tal et al. (1990) Agron. J. 82, 989–995; Gurevitch et al. (1990) J. Ecol. 78, 727–744; Koide (1991) Oecologia 85, 389–395; Latimer (1991) HortScience 26, 124–126; Martini et al. (1991) J. Am. Soc. Hort. Sci. 116, 439–445; Simpson (1991) North. J. Appl. For. 8, 160–165; Thomas and Strain (1991) Plant Physiol. 96, 627–634; Dubik et al. (1992) J. Plant Nutr. 15, 469–486; NeSmith et al. (1992) J. Plant Nutr. 15, 2763–2776; Samuelson and Seiler (1992) Env. Exp. Bot. 32, 351–356; Beeson (1993) J. Amer. Soc. Hort. Sci. 118, 752–756; McConnaughay et al. (1993) Oecologia 94, 550–557; NeSmith (1993) J. Plant Nutr. 16, 765–780; Ismail et al. (1994) Aust. J. Plant Physiol. 21, 23–35; Menzel et al. (1994) J. Hortic. Sci. 69, 553–564; Nobel et al. (1994) Physiol. Plant. 90, 173–180; Ran et al. (1994) Agron. J. 86, 530–534; Barrett and Gifford (1995) Aust. J. Plant Physiol. 22, 955–963; Liu and Latimer (1995) HortSci. 30, 242–246; Mandre et al. (1995) J. Amer. Soc. Hort. Sci. 120, 228–234; Agyeman et al. (1996) Ghana J. For. 2, 14–24; Hsu et al. (1996) HortSci. 31, 1139–1142; Huang et al. (1996) Plant and Soil 178, 205–208; Ismail and Noor (1996) Sci. Hortic. 66, 51–58; McConnaughay et al. (1996) Ecol. Appl. 6, 619–627; Giannina et al. (1997) Acta Hortic. 463, 135–140; Van Iersel (1997) HortSci. 32, 1186–1192; Will and Teskey (1997) Tree Physiol. 17, 655–661; Houle and Babeux (1998) Can. J. Bot. 76, 1687–1692; Nishizawa and Saito (1998) J. Amer. Soc. Hort. Sci. 123, 581–585; Ray and Sinclair (1998) J. Exp. Bot. 49, 1381–1386; Boland et al. (2000) J. Amer. Soc. Hort. Sci. 125, 135–142; Centritto (2000) Plant Biosystem. 134, 31–37; Haver and Shuch (2001) Plant Growth Reg. 35, 187–196; Yeh and Chiang (2001) Sci. Hortic. 91, 123–132; Aphalo and Rikala (2003) New Forests 25, 93–108; Loh et al. (2003) Urban For. Urban Green 2, 53–62; Ronchi et al. (2006) Funct. Plant Biol. 33, 1013–1023; Arizaleta and Pire (2008) Agrociencia 42, 47–54; Chirino et al. (2008) For. Ecol. Man. 256, 779–785; Climent et al. (2008) Silvae genetica 57, 187–193; Goreta et al. (2008) HortTechnology 18, 122–129; Kurunc and Unlakara (2009) New Zeal. J. Crop and Hortic. Sci. 37, 201–210; Oztekin et al. (2009) J. Food Agric. Env. 7, 364–368; Climent et al. (2011) Eur. J. Forest Res. 130, 841–850; Nord et al. (2011) Funct. Plant Biol. 38, 941–952; Mommer and De Kroon (pers. comm.); Pierik (pers. comm.).
B. Hydroponically grown plants
Richards and Rowe (1977) Ann. Bot. 41, 729–740; Carmi et al. (1983) Photosynthetica 17, 240–245; Tschaplinski and Blake (1985) Physiol. Plant. 64, 167–176; Hameed et al. (1987) Ann. Bot. 59, 685–692; Peterson et al. (1991) J. Exp. Bot. 42, 1233–1240; Thomas (1993) Plant Growth Reg. 13, 95–101; Ternesi et al. (1994) Plant Soil 166, 31–36; Bar-Tal et al. (1995) Sci. Hortic. 63, 195–208; Bar and Pressman (1996) J. Amer. Soc. Hort. Sci. 121, 649–655; Kharkina et al. (1999) Physiol. Plant. 105, 434–441; Xu et al. (2001) J. Plant Nutr. 24, 479–501.
Appendix 2
For this meta-analysis we screened the literature of the last 100 years. A total of 63 publications plus two additional unpublished experiments dealt with plants grown in pots of various sizes, 11 with plants grown in hydroponics with different levels of root confinement. The references are listed in Appendix 1. As we were interested in not only the physical aspect of container volume, but also in the resources that come with it, we compared pot size treatments based on size, including the possible additional benefits of increased nutrient and water availability. The experiments with hydroponically-grown plants are not included in the main analysis. Differences in the shape of the pots were not independently analysed either. In several publications only pot diameter was reported. For a sample of 30 different pots ranging in diameter (Ø) from 7 to 40 cm we derived an estimate for pot volume (V) based on the empirical equation V = π(Ø/2)2 × (0.46 + 0.8397 × Ø – 0.002307 × Ø2). If additional data were missing, we assumed pots to be filled with substrate up to the rim.
For each species or genotype in a given experiment, we determined the biomass at the last harvest and separated this variable in biomass of leaves, stems, roots and reproductive mass as far as data were provided. To capture the importance of pot size for growth we calculated for each experiment the proportional increase in total plant dry mass relative to the proportional increase in pot size, by calculating the slope of the lines that were fitted through the observed log-transformed plant masses and pot volumes, separately for each species in each experiment. Because experiments differed in the range of pot size used, we scaled these slopes in such a way that the number reflects the percent change in biomass (or another variable) given a doubling in pot size (see Appendix 3 for more details). For a more detailed analysis, log-transformed data from experiments which included three or more pot sizes were also fitted with a saturating equation, as described in Appendix 3 and calculated with the nls procedure in R (R Development Core Team 2011).
Appendix 3
Let V1 and V2 be pot volume 1 and 2 and B1 and B2 the total plant dry biomass that is observed at the respective pot volumes: because proportional differences are the focus of interest, we calculate the slope of the line that connects these points as:

Suppose we want to know the fraction f by which plant mass increases when pot size doubles. Then

and

Assuming a common slope s over the whole trajectory of pot masses considered, this results then in
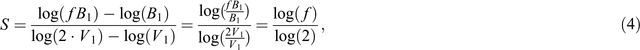
and, after rearrangement

The same procedure applies if slope s is determined by linear regression through more than two points.
To relate the slope of the line to the observed values of total plant mass per unit pot volume (BVR), we took the following approach: In cases where an experiment consisted of two pot sizes, a linear regression as above was calculated and the resulting slope attributed to both points. In case an experiment consisted of more than two pot sizes and a second order polynomial showed no significant saturating trend, we fitted a straight line through all points. Otherwise, a saturating curve was fitted through the points, of the form

with the constraint that a, b and c should be positive. The slope of the line at each pot size is then given by the derivative:

After the slope and f were calculated for each pot volume in each experiment, data were binned in five categories of BVR.
Appendix 4.
We measured the root distribution of Hordeum vulgare (barley) and Beta vulgaris (sugar beet), grown in a glasshouse in cylindrical containers with a volume of 1.3 L (length 26 cm, inner diameter 8 cm). Measurements were done by non-destructive imaging of roots using nuclear magnetic resonance imaging (MRI) as described extensively by Jahnke et al. (2009). This method is able to detect roots with a diameter down to 300 μM, which implies that fine roots go unnoticed. Image segmentation of the root system was done by thresholding the image and removing the stem and the sugar beet, with the RegionGrowingMacro module in MeVisLab (ver. 2.2.1; MeVisLab, Bremen, Germany). After segmentation, the pixel values were integrated for the inner and outer regions of the soil core and divided by the integral over the whole pot.