Ripening of grape berries can be advanced or delayed by reagents that either reduce or increase ethylene levels
Christine Böttcher A , Katie E. Harvey A , Paul K. Boss A and Christopher Davies A BA CSIRO Plant Industry, PO Box 350, Glen Osmond, SA 5064, Australia.
B Corresponding author. Email: christopher.davies@csiro.au
Functional Plant Biology 40(6) 566-581 https://doi.org/10.1071/FP12347
Submitted: 19 November 2012 Accepted: 23 February 2013 Published: 5 April 2013
Abstract
Grape (Vitis vinifera L.) berries are considered to be nonclimacteric fruit as they do not exhibit a large rise in ethylene production or respiration rate at the onset of ripening (veraison). However, ethylene may still play a role in berry development and in ripening in particular. (2-Chloroethyl)phosphonic acid (CEPA), an ethylene-releasing reagent, delayed ripening when applied early in berry development. In agreement with a role for ethylene in controlling the timing of ripening, the application of an inhibitor of ethylene biosynthesis, aminoethoxyvinylglycine (AVG), advanced ripening, as did abscisic acid, when applied during the preveraison period. Applications of CEPA nearer to the time of veraison enhanced berry colouration. Changes in the expression of ethylene biosynthesis and receptor genes were observed throughout berry development. Transcript levels of some of these genes were increased by CEPA and decreased by AVG, suggesting changes in ethylene synthesis and perception during the preveraison period that might contribute to the biphasic response to CEPA (ethylene). The significant delay of ripening in field-grown grapes through the application of CEPA also indicates that this may be useful in controlling the timing of veraison, and therefore harvest date, in warmer climates.
Additional keywords: (2-chloroethyl)phosphonic acid, aminoethoxyvinylglycine, veraison, Vitis vinifera.
Introduction
Differential screening, cDNA and oligonucleotide microarray analysis and 2D protein gel analysis has shown that the levels of mRNAs for thousands of genes and their corresponding proteins change during grape (Vitis vinifera L.) berry ripening (Davies and Robinson 2000; Terrier et al. 2005; Waters et al. 2005; Deluc et al. 2007; Deytieux et al. 2007; Giribaldi et al. 2007; Pilati et al. 2007). Such widespread changes occur in other fruit as ripening commences, and plant growth regulators (PGRs) are thought to be involved in orchestrating these changes (Giovannoni 2004). Grapes are considered to be nonclimacteric fruit (Coombe 1973) and, by definition, nonclimacteric fruit are thought to lack the respiratory rise and the strong involvement of ethylene in the control of ripening that is seen in climacteric fruit.
The possible involvement of several PGRs in promoting grape berry ripening has been proposed. Abscisic acid (ABA) levels rise sharply around the time of veraison and the application of ABA during a certain period before veraison can advance the initiation of ripening (Coombe 1973; Coombe and Hale 1973; Hale and Coombe 1974; Inaba et al. 1976; Cawthon and Morris 1982; Davies et al. 1997; Zhang et al. 2003; Deytieux-Belleau et al. 2007; Wheeler et al. 2009). In addition, some of the genes expressed during the ripening phase are likely to be ABA-responsive (Deluc et al. 2007; Grimplet et al. 2007; Pilati et al. 2007). Brassinosteroids (BRs) have also been implicated in the control of grape berry ripening as BR levels, like those of ABA, rise sharply around veraison. Furthermore, BR application before veraison can advance ripening, whereas the application of a BR biosynthesis inhibitor delays it (Symons et al. 2006). Conversely, the application of other PGRs such as auxins can delay ripening (Weaver 1962; Hale 1968; Hale et al. 1970; Coombe 1973; Hale and Coombe 1974; Davies et al. 1997; Fujita et al. 2006; Deytieux-Belleau et al. 2007; Böttcher et al. 2011a, 2011b, 2012). Recently, the role of ethylene in nonclimacteric fruit ripening has been revisited. Although ethylene levels seem to be much lower than in climacteric fruit, there is evidence that small changes in ethylene and CO2 evolution may occur during ripening in some nonclimacteric fruit (Cazzonelli et al. 1998; Iannetta et al. 2006; Wang et al. 2007). Previous studies have investigated the possible role of ethylene in berry development (reviewed by Böttcher and Davies 2012) but the outcomes have been mixed. The low ethylene levels present in grape berries make reliable detection difficult, especially as fruit removed from the vine may differ in ethylene evolution compared with attached berries, and measurement of ethylene on the vine is technically problematic. Some reports indicate modest increases in ethylene levels around veraison (Alleweldt and Koch 1977; Düring et al. 1978; Chervin et al. 2004) but other reports describe no such increase (Coombe 1973; Weaver and Singh 1978). It is therefore possible that there is a small rise in ethylene levels sometime around veraison but there is general agreement that ethylene levels are low during the ripening phase. Changes in the level of ethylene receptors may also be involved in fruit ripening (Trainotti et al. 2005; Kevany et al. 2007, 2008). Changes in the transcript levels of some ethylene receptors have been observed during grape berry development (Chervin and Deluc 2010; Deluc et al. 2007) but no measures of receptor protein levels have been reported.
The treatment of berries with ethylene, either as a gas or through the application of the ethylene-releasing compound (2-chloroethyl)phosphonic acid (CEPA), and with inhibitors of ethylene perception has yielded somewhat mixed results. This could be due to any number of factors including the developmental stage of the fruit at application, varietal differences in uptake and response, the method of application and growth conditions. There are many reports where CEPA application has increased colour development, with or without a concomitant increase in sugars, in a wide range of grape cultivars (reviewed by Szyjewicz et al. 1984). The increase in anthocyanin levels is probably through an increase in the transcript levels and enzyme activities of several genes involved in anthocyanin synthesis (Roubelakis-Angelakis and Kliewer 1986; El-Kereamy et al. 2003; Tira-Umphon et al. 2007; Chervin et al. 2009). Other studies using 1-methylcyclopropene (1-MCP), an ethylene receptor antagonist, provide further support for the involvement of ethylene during berry ripening. 1-MCP treatment at the appropriate time can reduce the expression of ripening-associated genes and delay various ripening-related processes (Chervin et al. 2004, 2009).
Interestingly, there are also reports of CEPA and ethylene delaying ripening. Although CEPA and ethylene treatments around the time of veraison appear to advance ripening, earlier treatments can delay it (Hale et al. 1970; Coombe 1973). Therefore, CEPA (and, by inference, ethylene) can be considered to be an inhibitor or promoter of ripening, depending on the developmental stage of the berries at the time of application (i.e. the response to ethylene appears to be biphasic). Changes in the response to ethylene during development have also been reported in citrus, another nonclimacteric species (Katz et al. 2004).
Many questions about the involvement of ethylene in grape berry development remain unanswered. In this paper, experiments are described where ABA, CEPA and aminoethoxyvinylglycine (AVG) were applied to grape bunches and their effect on ripening was monitored. It appears that ethylene may act to retard berry development and, as a consequence, ripening when applied at a particular preveraison stage. Conversely, reducing the level of ethylene biosynthesis during the preveraison period through AVG application can hasten ripening.
Materials and methods
Treatment of field-grown fruit with PGRs
PGR treatments of grape (Vitis vinifera L.) berries were conducted over two seasons. In the initial study, during the 2006–07 season, bunches of V. vinifera cv. Cabernet Sauvignon vines, grown at Charleston, Adelaide Hills, South Australia (Nepenthe Wines, 34°92′S, 138°92′E) were sprayed with four different treatments: control: (0.05% (v/v) Tween 20 (Pharmacia LKB Biotechnology, Uppsala, Sweden)); AVG: (ReTain, Valent BioSciences, Libertyville, IL, USA; 1.67 g L–1, 0.05% (v/v) Tween 20); ABA: ((+)cis, trans-ABA (AG Scientific, San Diego, CA, USA)); 400 mg L–1, 0.05% (v/v) Tween 20) and CEPA as Ethrel (Bayer CropScience, East Hawthorn, Vic., Australia; 300 µL L–1, 0.05% (v/v) Tween 20). ABA was taken up in 500 µL of 100% ethanol before dissolving in the Tween solution. The same bunches were sprayed on two occasions during the preveraison period, 17 January 2007 (12 days before veraison) and 24 January 2007 (5 days before veraison). The trial was of a randomised triplicate design with control, ABA, AVG and CEPA treatments randomised over three adjacent rows. Each replicate consisted of 25 bunches selected to be of a similar size and age from four or five vines, with untreated vines separating the different treatments (one vine for all treatments except CEPA, where three untreated vines were used to separate treatments). Sprays were applied to run off using hand-held sprayers; drift onto other parts of the vine was prevented by the use of a plastic shield. Bunches on both sides of north–south oriented rows were sprayed.
Measurements of berry weight (80 berries per replicate), total soluble solids (TSS) and anthocyanin levels were taken at regular intervals to measure the progress of berry development. TSS were measured as °Brix in individual berries using a refractometer. To prepare samples for total anthocyanin determination, grape berries, frozen in liquid nitrogen, were ground to a powder using an IKA A11 basic analytical mill (IKA, Staufen, Germany) and 0.1 g of powdered sample was added to 1 mL of methanol containing 1% (v/v) HCl. The anthocyanins were extracted at room temperature in the dark on a rotating mixer for 1 h. The tissue was pelleted by centrifugation at 18 000×g for 15 min and the supernatant was retained. Samples were diluted as required and total anthocyanin levels were estimated spectrophotometrically by reading absorbance at 520 nm immediately following centrifugation.
The results from the above experiment were used to design a more expansive study during the following season (2007–08) to investigate the effect of treatment timing on changes in berry development. V. vinifera cv. Shiraz fruit grown at two different sites were sprayed. At a vineyard in Hahndorf, Adelaide Hills, South Australia (Nepenthe Wines, 35°02′S, 138°84′E), three sprays were completed during the preveraison period, 7 January 2008 (22 days before veraison), 17 January 2008 (12 days before veraison) and 22 January 2008 (7 days before veraison). In this study, separate vines were used for each of the three treatment dates. The rest of the experimental design and applications were as described above, except that TSS was measured for a combined sample from each of the replicates rather than for individual berries, and that CEPA as Ethrel was used at 600 µl L–1. Berries were sampled 24 h after each CEPA treatment and RNA was extracted to study its effects on short-term gene expression by quantitative real-time PCR analyses (qPCR).
At the Willunga site, South Australia (Chalk Hill Wines, 35°26′S, 138°55′E), V. vinifera cv. Shiraz fruit spread over six east–west oriented rows were sprayed as described for the Hahndorf site, except that four sprays, each on a different set of vines, were completed on 5 December 2007 (29 days before veraison), 17 December 2007 (17 days before veraison), 27 December 2007 (7 days before veraison) and 3 January 2008 (at veraison).
Ex planta testing of changes in gene expression in response to AVG
To test the effect of sucrose and AVG on ethylene biosynthesis and receptor gene expression, Shiraz berries were sampled from 40 bunches from 10 vines at two time points before veraison (21 days and 6 days, 8 January 2008, 23 January 2008 respectively) at the Hahndorf site, sterilised in 0.05% (v/v) Tween 20 containing one Milton tablet L–1 (Milton Australia, Laverton North, Vic., Australia) for 1 h and washed three times with sterile nanopure water. All following procedures were carried out in a laminar flow under sterile conditions. A thin slice of each berry was cut off around the brush area to ensure good contact with the agarose medium and 20 berries were placed on Petri dishes containing 30 mL of Gamborg’s media, 0.025% (w/v) casein hydrosylate, 0.8% (w/v) agar, pH 5.7–5.8 and one or both of the following additives (final concentrations): ReTain (Valent BioSciences; 125 mg L–1 AVG, filter sterilised), 12% (w/v) sucrose. Berries were placed on the plates with the cut surface facing the agar, the plates were sealed with Parafilm (Bemis Company Inc., Neenah, WI, USA) and kept in the dark at 25°C. After 20 h, the berries were harvested and frozen in liquid nitrogen.
Grape berry developmental series
A series of berry samples were taken from flowering to ripeness to provide a developmental series for the analysis of gene expression. Shiraz vines grown at Willunga (as above) were used. Fruit samples were collected during the 2007–08 growing season. Flowering was defined as the time when ~50% of the opercula had fallen from the flowers (50% cap-fall). Samples were collected from both sides of vines in north–south oriented rows. Berries (100 per sampling time) were sampled randomly from all bunches in all parts of the canopy. The berries were deseeded before being frozen in liquid nitrogen. All samples were stored at −80°C until required.
Preparation of RNA and cDNA synthesis
Grape RNA was isolated essentially as described by Davies and Robinson (1996). The RNA was further purified by precipitating with 0.44 vol of 10 M LiCl at −20°C for 3 h. After centrifugation and washing with 70% (v/v) ethanol, the pellet was dried and resuspended in 10 mM TRIS-HCl (pH 7.5), 1 mM EDTA. Total RNA was quantified using a spectrophotometer (Nanodrop 1000; Thermo Fisher Scientific, Wilmington, DE, USA; absorbance at 260 nm) and 100 μg was further purified and DNase-treated using RNeasy mini spin columns (Qiagen, Hilden, Germany) and the RNase-Free DNase set (Qiagen) as described by the manufacturer. One μg of purified RNA was run on an ethidium bromide-stained agarose gel in 20% (v/v) formaldehyde buffer to check for quality. Complementary DNA was synthesised in 20- μL reactions from 2 μg of total RNA using the SuperScript First-strand cDNA synthesis system (Invitrogen, Carlsbad, CA, USA) and the 3′-end, (dT)17-adpater primer (Frohman et al. 1988) according to the manufacturer’s instructions. Complementary DNA reactions were diluted 10-fold before use in qPCR.
qPCR analysis
Expression of 1-aminocyclopropane-1-carboxylate synthase (ACS), 1-aminocyclopropane-1-carboxylate oxidase (ACO) and ethylene receptor genes was determined by qPCR using a Rotor Gene 3000 system (Corbett Research, Mortlake, NSW, Australia). The primers used were: ACS genes: ACS1RT-F (AM426886) 5′-GAACTTTGTGCTTAAAGCCAAAGAA-3′, ACS1RT-R 5′-GCGGCTCATCCAATCCTCTG-3′ (104 bp), ACS2RT-F (AM429962) 5′-GGCTCTTTGGAACTCCATCCT-3′ and ACS2RT-R 5′-TAGCCACTGCTCCCATCTCC-3′ (125 bp); ACO genes: ACO1RT-F (AY211549) 5′-GGTGGAGAAAGAAAAGGAGACA-3′, ACO1RT-R 5′-TCTGTTGGCAAAGGACTCAA-3′ (179 bp), ACO2RT-F (CB970406) 5′-CTGGAGAAAGAAGCAGAGAACGATC-3′, ACO2RT-R 5′-TGGAAACAAGCAACATAAATCCTTC-3′ (205 bp), ACO3RT-F (AM427708) 5′-GCTCCCAAGCTCTTATATCCAGATC-3′ and ACO3RT-R 5′-GCATGAAGACTGTGATGCCCA-3′ (137 bp); ethylene receptor genes: ETR1RT-F (AF243474) 5′-AGCACCGTTTTCTGTTTGAGACC-3′, ETR1RT-R 5′-CCACAATATTTCTGCATTGCCG-3′ (133 bp), ETR2RT-F (CB975799) 5′-TCTGCTGGATGGAATTGCTGAG-3′, ETR2RT-R 5′-CTATGCCGCAAGCTGGATGT-3′ (180 bp), EIN4RT-F (CB342656) 5′-TGAAGCGAGCCAACGATGG-3′, EIN4RT-R 5′-GCTTGAATGGAGTTCAACAAATCG-3′ (138 bp), ERS1RT-F (CD799344) 5′-TCCAAATGAACCATCAGCGC-3′, ERS1RT-R 5′-TTGCATACCTTGGCACTCGG-3′ (186 bp).
Each reaction contained 1× SYBR Green PCR Master Mix (Life Technologies Corporation, Carlsbad, CA, USA), 2 μL of diluted cDNA, each primer at 333 nM and water to 20 μL. Cycling conditions were: 95°C for 10 min and 40 cycles: 95°C for 30 s, 58°C for 30 s and 72°C for 30 s, followed by a melt cycle of 1°C increments from 50°C to 96°C (45 s for the first step; 5 s for each subsequent step). Each primer pair gave a single product of the expected size and sequence, verified by analysis of the melt curve, agarose gel electrophoresis and DNA sequencing. All reactions were performed in triplicate. The preparation of standard DNA for the calibration curves was done as described by Böttcher et al. (2010). The expression value of each gene in each cDNA tested, which was determined by reference to the standard curve, was normalised to the level of expression of Actin2 (primers designed to CF208516 forward 5′-GGGCCAGGCTATTGCAACTC-3′; reverse 5′-GCATCACCAATCACTCTCCTGC-3′).
Statistical data analysis
The significance of any differences between treatments was tested by Student’s t-test or ANOVA with Duncan’s post hoc test, using SPSS ver.16.0 (SPSS Inc., Chicago, IL, USA).
Results
Field experiments during the 2006–07 season – effects of PGRs on berry ripening
A preliminary study was conducted during the 2006–07 growing season to test the effects of various PGRs on the ripening of grape berries. The date of veraison for control fruit was 29 January 2007. The results indicated that ABA and AVG treatments advanced ripening, but CEPA treatment delayed ripening as measured by changes in TSS levels. CEPA treatment during the preveraison period delayed the accumulation of sugars (Table 1), as TSS levels for the CEPA-treated fruit were significantly lower than those for the control fruit for the last three samples (35, 50 and 64 days after initial spraying (DAIS)). No significant differences in berry weight were detected between CEPA-treated and control fruit (Table 1). Anthocyanins were only measured for the harvest samples and no significant differences were recorded between the treatments (Table 1).
The TSS values of ABA-treated fruit were higher than those for control fruit between 22 and 50 DAIS (Table 1), and ABA-treated fruit were significantly heavier than control fruit at 12 and 22 DAIS (Table 1).
Although the weight of AVG-treated fruit was not significantly different compared with that of the control fruit, the TSS levels of AVG-treated fruit were significantly higher than those for the control fruit at all sampling times (Table 1), indicating that, like ABA, AVG can advance the timing of sugar accumulation when applied to berries during the preveraison period.
Field experiments during the 2007–08 season – effects of PGRs on berry ripening
In response to the effects of the treatments on berry ripening described above, it was decided to conduct a more extensive experimental program to confirm and further expand on these results during the following season. To this end, treatments were applied at several different times in relation to the date of veraison at two sites that had different climatic conditions (Gladstones 1992). There were numerous differences in the effects of the different treatments, and all the data are given in Tables 2 and 3. In an effort to simplify the presentation of the results and discussion, the focus is on the trends that are thought to be the most important and that are best supported by evidence of statistical significance. Although the differences between the treatments when compared with the control were not always statistically significant, the differences between ABA- and AVG-treated fruit compared with CEPA-treated fruit were more frequently so. This can be attributed to these treatments having opposite effects (see below).
As seen in the 2006–07 experiment, CEPA, when applied at certain times during the preveraison period of development, delayed ripening in the experiments at both field sites during the 2007–08 season. At the Willunga site, CEPA Spray 1 (5 January 2007, 29 days before veraison) and Spray 2 (17 January 2007, 17 days before veraison) both resulted in a delay in the increase of TSS and anthocyanin levels (Table 2). The effect on TSS levels was greater for Spray 2 than Spray 1. The date of veraison for control fruit was 3 January 2008. There were no significant berry weight differences between the control and CEPA-treated fruit for these samples (Table 2). CEPA Spray 1 (7 January 2008) at the Adelaide Hills site resulted in a similar delay in the accumulation of TSS and anthocyanins (Table 3). However, in contrast to the effect seen at Willunga, the CEPA Spray 1 treatment at the Adelaide Hills site resulted in a significant delay in berry size increase (Table 3). The date of veraison at the Adelaide Hills site for control fruit was 29 January 2008. Later CEPA sprays (Willunga Sprays 3 (7 days before veraison) and 4 (at veraison), and Adelaide Hills Spray 3 (7 days before veraison)) at both sites seemed to have little or no delaying effect on any of the ripening parameters (Tables 2 and 3).
The above results indicated that CEPA treatments applied earlier during development delayed the entry of the fruit into the ripening phase. In contrast, the third spray at the Adelaide Hill site, which was applied only a short time (7 days) before the veraison date for control fruit, appeared to enhance anthocyanin accumulation. The CEPA-treated fruit had higher anthocyanin levels than the control berries at 7, 14 and 24 DAIS (Table 3). There were no significant differences between control and CEPA-treated fruit in regards to TSS levels and berry weight (Table 3). Anthocyanins were not measured for the later sprays at the Willunga site.
In contrast to the effects observed during the 2006–07 experiment, neither ABA nor AVG treatments appeared to greatly alter the onset or progression of ripening during the trial conducted during the 2007–08 season at Willunga (Table 2). However, in the Adelaide Hills experiment, the treatment of berries with ABA or AVG advanced ripening (Table 3). Although there were some minor differences between the effects of ABA and AVG during the Adelaide Hills 2007–08 experiment, the general trends were similar in that both tended to enhance ripening when applied at a particular time during the preveraison period (i.e. Spray 2 on 17 January 2008; Table 3). ABA-treated fruit had significantly higher TSS levels at 12 DAIS than control fruit, whereas AVG-treated fruit had a higher TSS value at 40 DAIS. Berry weights for ABA- and AVG-treated fruit were significantly higher than control berry weights at 12 DAIS (Table 3), but the major effect was on anthocyanin levels. ABA- and AVG-treated fruit had higher levels of anthocyanins than control fruit at 12, 19 and 29 DAIS, with ABA-treated fruit also having higher levels at 40 DAIS.
Expression of ethylene synthesis and perception genes during berry development
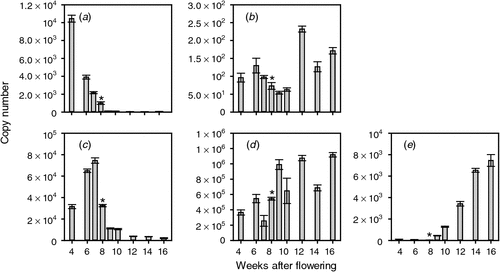
Given that the biosynthesis and perception of ethylene during the preveraison phase seems to be of particular interest, it was decided to measure the expression of grapevine genes likely to be involved in these processes. An extensive search was made of the grape expressed sequence tags and genome sequences to identify the ACS and ACO genes in grapevine. Primers sets were made to all candidates and qPCR analysis was conducted. Only those genes with detectable expression during berry development are reported here. ACS catalyses the first committed step in ethylene synthesis and of the six putative ACS genes, two were found to be expressed in Cabernet Sauvignon berries. One gene (ACS1) was significantly expressed during the preveraison phase. Its mRNA levels were highest in young berries (copy number = 10 450 at 4 weeks after flowering (WAF)) then decreased steadily to be at low levels for the rest of berry development (copy number = 49 at 12 WAF; Fig. 1). A second ACS gene (ACS2) was expressed throughout berry development (Fig. 1). Its level of expression was much lower than that of ACS1 in young berries (copy number = 96 at 4 WAF) but after veraison, the expression of ACS2 increased and was higher (copy number = 232 at 12 WAF) than that of ACS1. The second and final step of ethylene synthesis is catalysed by ACO, and three of the four putative ACO genes were expressed during berry development. ACO1, like ACS1, was expressed most highly during the preveraison period but the highest levels were observed in berries 6 and 7 WAF. The levels then decreased to relatively low levels at veraison and there was low expression throughout ripening (Fig. 1). The ACO2 gene was expressed throughout berry development, with generally higher levels during the period after veraison (Fig. 1). The levels of this mRNA were ~10-fold higher than that for ACO1. The expression of the other ACO gene, ACO3, was very low before veraison but increased steadily after veraison to be at a maximum level at 16 WAF (Fig. 1).
|
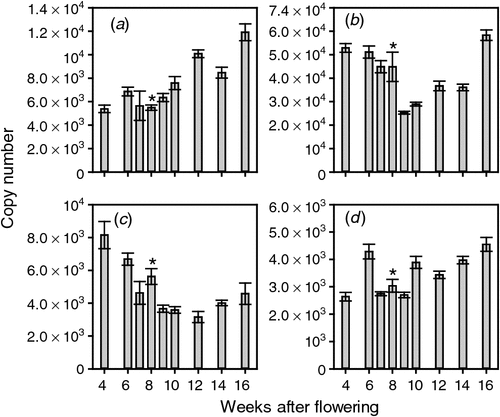
Although the biosynthesis of ethylene is important in controlling its levels in vivo, the berries’ response to ethylene can also be moderated through the perception pathway. The first step in ethylene perception and signalling is the binding of ethylene to its receptor proteins. To test whether the ethylene signal is modified by changes in receptor mRNA levels, the transcript levels of the four reported putative ethylene receptors (Chervin and Deluc 2010) were assayed throughout berry development. All four putative ethylene receptor-encoding genes were expressed throughout berry development. ETR1 levels were constant before veraison but increased gradually after veraison to be highest at 16 WAF (Fig. 2). ETR2 levels were highest in young fruit and in the last sample taken (16 WAF) with somewhat lower expression levels in fruit 9–14 WAF (Fig. 2). ETR2 was the most highly expressed of the putative ethylene receptor genes throughout berry development. The expression of ERS1 was highest in berries 4 WAF, after which it decreased steadily until veraison. The level of ERS1 mRNA changed little throughout ripening (Fig. 2). EIN4 levels were reasonably constant throughout berry development (Fig. 2).
|
Changes in ethylene biosynthesis and receptor gene transcription in response to CEPA application
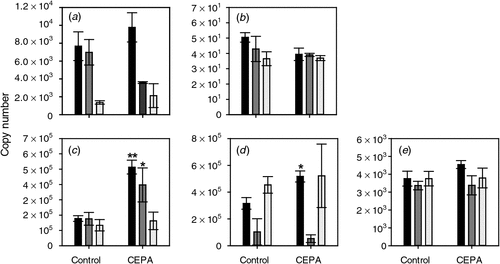
The ethylene biosynthesis and signalling pathways are known to be responsive to changes in ethylene levels (reviewed by Lin et al. 2009). The responsiveness of these pathways in grape berries was tested at the Adelaide Hills site by sampling CEPA-sprayed berries. The expression of ACS1 and ACS2 was not significantly altered by any of the CEPA treatments (Fig. 3).
|
The expression of ACO1 was relatively constant in the Tween-treated control samples at all three timepoints. In contrast, expression was significantly induced by CEPA treatment by the two earlier sprays, i.e. 7 January 2008 and 17 January 2008 (Fig. 3). Interestingly, there was no significant induction by the spray closest to veraison (22 January 2008, 7 days before veraison). ACO2 expression was also significantly induced by the earliest CEPA spray (7 January 2008). None of the three CEPA treatments caused a significant change in ACO3 expression (Fig. 3).
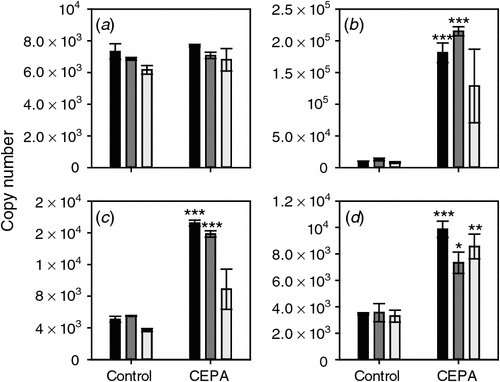
The response of the four putative ethylene receptor genes to CEPA was also tested. ETR2, ERS1 and EIN4 all showed a clear induction by CEPA sprays 1 (7 January 2008) and 2 (17 January 2008) but only EIN4 was significantly induced by the third spray (22 January 2008) (Fig. 4).
|
Changes in ethylene biosynthesis and receptor gene transcription after AVG and sucrose application to ex planta berries
To test the effect of lowered ethylene biosynthesis on the expression of ethylene biosynthesis and receptor genes, berries were harvested at the Adelaide Hills site at two timepoints before veraison (8 January 2008 and 23 January 2008) and subjected to AVG application on agar plates. The ex planta methodology used was designed to ensure that the berry was rapidly exposed to a constant level of AVG throughout the experiment. The berries were harvested for RNA extraction after a 20-h incubation period. The expression patterns for the ethylene biosynthesis pathway genes were quite complex. ACS1 expression was not affected by any of the treatments in berries treated on 23 January 2008 (6 days before veraison); however, its expression was significantly reduced by both AVG and sucrose in berries treated earlier during development (8 January 2008; 21 days before veraison) (Fig. 5). ACS2 expression was very low and not significantly affected by any of the treatments. ACO1 expression was suppressed by AVG treatment and induced by sucrose at both berry developmental stages (Fig. 5). In contrast, ACO2 expression was slightly induced by AVG treatment but repressed by sucrose at both timepoints (Fig. 5). The expression of the ACO3 gene, which is primarily expressed after veraison, was slightly reduced by AVG in the berries at 21 days before veraison, but not in the berries 6 days before veraison. However, its expression was induced markedly by sucrose at both timepoints (Fig. 5).
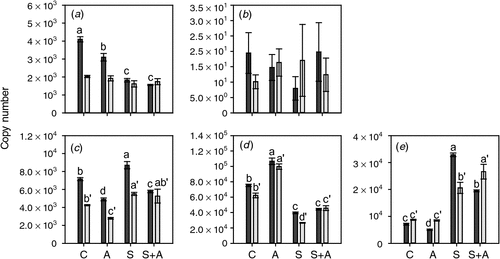
|
AVG treatment affected the expression of the three ethylene-responsive ethylene receptor genes in the opposite manner to CEPA application. The mRNA levels of ETR2, ERS1 and EIN4 (Fig. 6) were all significantly decreased at both timepoints by AVG treatment (Fig. 6). Sucrose had only minor effects on the expression of these three genes when compared with the changes resulting from exposure to AVG. ERS1 expression was not affected by sucrose, ETR2 expression was slightly induced at both stages of berry development and EIN4 expression was increased somewhat at the earlier developmental stage (21 days before veraison) (Fig. 6). ETR1 was much less responsive to the AVG treatment than the other three genes and mRNA levels were slightly increased by AVG in berries at the earlier stage of development. Sucrose induced relatively small increases in ETR1 expression at both stages of berry development (Fig. 6).
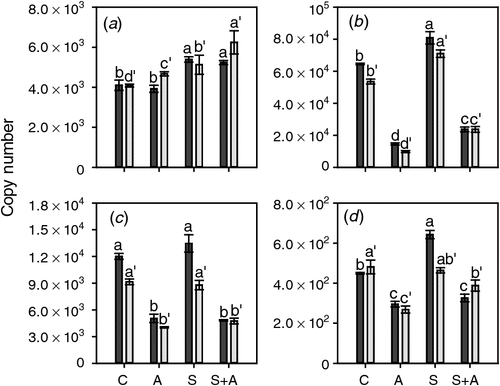
|
Discussion
CEPA can inhibit or promote ripening, depending on the berries’ developmental stage at application
One of the striking results from this study was the delay of TSS and anthocyanin accumulation in grape berries treated with CEPA at certain times during the preveraison period. The timing of application relative to the stage of berry development was crucial to the outcome of CEPA application. The treatment of berries with CEPA inhibited ripening when applied sufficiently early. The TSS level for CEPA-treated fruit was significantly lower than for the control fruit in the last three samples taken during the 2006–07 experiment (Table 1). Similarly, the first two CEPA treatments at the Willunga site during the 2007–08 season significantly delayed increases in TSS and anthocyanin levels (Table 2) without a significant effect on berry weight. The third spray delayed the TSS increase at one sampling date without affecting berry weight, and the last spray increased berry weight at one sampling date without affecting TSS levels. A delay in berry ripening was also observed at the Adelaide Hills site, but with variations on the effects seen at the Willunga site. At the Adelaide Hills site, TSS, anthocyanin and berry weight increase were significantly delayed by the first spray (Table 3).
The current study confirms and extends the results of two previous and less extensive studies where a delay in ripening was observed after CEPA application (Hale et al. 1970; Coombe and Hale 1973). The delay in ripening caused by CEPA is in contrast to many reports where CEPA application nearer the initiation of ripening caused an increase in colour and, on some occasions, sugar levels (see reviews by Szyjewicz et al. (1984) and Böttcher and Davies (2012)). Indeed, in the experiment at the Adelaide Hills site, we also observed that CEPA treatment significantly increased colour accumulation at three sampling timepoints when applied a week before veraison (Table 3).
Only bunches were sprayed in the current study, showing that the observed delay in ripening can be achieved by local application. However, it is possible that the diffusion of CEPA or ethylene to other parts of the plant may have resulted in some secondary effects. CEPA sprayed at the concentration used in these experiments onto the vegetative tissue had no visible effect on vine health (data not shown).
The delaying of ripening caused by the application of CEPA during the earlier preveraison period could be due to an effect on berry growth. There is increasing evidence from a range of plant species for a biphasic response to ethylene, where high levels inhibit growth and low levels stimulate growth (Pierik et al. 2007). The application of CEPA is expected to have resulted in the evolution of a high level of ethylene, probably much higher than that normally present in grape berries. These high levels may temporarily have slowed berry growth, resulting in a delay in veraison. CEPA application nearer to the time of veraison may not have an inhibiting effect, as the fruit may have developed sufficiently for ripening to proceed. However, much of the evidence from the current study is consistent with CEPA treatment having little effect on berry growth. The first three sprays at Willunga resulted in a significant reduction in the accumulation of TSS and anthocyanins at one or more sample points, but no significant differences in berry weight were observed (Table 2). The first spray at the Adelaide Hills site resulted in delayed berry weight increase as well as delayed TSS and colour accumulation, but the third spray, which caused an increase in colour, was not accompanied by any change in berry weight (Table 3). The delay in ripening may therefore result from a direct effect on the initiation of ripening rather than on an inhibition of growth. Due to the interactions reported between ethylene signalling and the various other PGR signalling pathways (Lin et al. 2009), the effect of ethylene on berry development may be a secondary response resulting from ethylene-induced changes in the concentration or perception of other PGRs.
The increase in anthocyanin accumulation arising from CEPA Spray 3 at the Adelaide Hills site (Table 3) is in line with previous studies showing that CEPA and ethylene can increase the activity of the anthocyanin biosynthesis pathway, resulting in higher anthocyanin levels (Szyjewicz et al. 1984; Roubelakis-Angelakis and Kliewer 1986; El-Kereamy et al. 2003; Tira-Umphon et al. 2007; Chervin et al. 2009).
ABA treatment promotes ripening
The data presented in this paper provide further confirmation that ABA application during a period of a few weeks before veraison can promote ripening. Previous studies have shown that ABA applied within a few weeks of veraison can advance the increases in TSS, colour and berry size associated with ripening (reviewed by Böttcher and Davies 2012). However, there is considerable variation between the reports regarding which processes ABA can influence and, in some cases, there is a lack of statistical evidence.
During the 2006–07 season, the treatment 9 days before veraison (repeated at 2 days before veraison) promoted an increase in TSS as measured by changes in Brix (Table 1). Similarly, ABA sprayed 12 days before veraison (Spray 2) in the Adelaide Hills during the 2007–08 season seemed to be effective in promoting ripening, as ABA-treated fruit had significantly higher TSS, anthocyanin levels or weight at particular stages (Table 2). The changes in all three measured parameters suggest that the entire ripening process had been accelerated, not just one component of it. Treatment earlier than this, at 22 days before veraison (Spray 1), or later (Spray 3, 7 days before veraison) did not seem as effective (Table 1). The influence of developmental stage on the effect of applications indicates that the ability of the berry to perceive and respond to the applied growth regulators changes during development (as described above for CEPA). Such changes in response to PGRs during development may explain some of the wide variation seen in published reports regarding the effect of exogenous ABA on berry ripening (reviewed by Böttcher and Davies 2012).
AVG can promote ripening
AVG applied during the preveraison period had a similar effect to ABA in that it also promoted berry ripening. The times when the application of AVG advanced ripening most effectively appeared to be the same as those for ABA and, as with ABA, the effects were modest. There was no evidence from the current experiments that AVG applications applied later in the preveraison period, up to within a week of veraison, significantly delayed ripening. This is in contrast to the inhibition of ripening observed when 1-MCP was used to block ethylene perception by receptors (Chervin et al. 2004). The reasons for this apparent difference are unclear but may relate to the different mode of action of these two inhibitors. AVG acts as a competitive inhibitor of ACS activity, thereby preventing ethylene biosynthesis (Boller et al. 1979; Yu et al. 1979; Yamagami et al. 2003) but 1-MCP acts as an inhibitor of ethylene perception (Sisler and Serek 2003). AVG might also affect auxin biosynthesis in berries (see below).
It is possible that the application of AVG to the whole vine rather than just to the bunches may be more effective in promoting ripening, as AVG levels in the berries may be diluted by its movement from the berries to other parts of the vine, and ethylene synthesised in the vegetative part of the plant may still be having some effect on the AVG-treated berries.
Apart from having a direct effect on ethylene synthesis, there may be another action of AVG involved in the hastening of veraison in AVG-treated berries. AVG was recently described as an inhibitor of a family of tryptophan aminotransferases in Arabidopsis thaliana (L.) Heynh. (Soeno et al. 2010). These aminotransferases are closely related to ACS proteins (reviewed by Theologis 1992) and catalyse the first committed step in a tryptophan-dependent biosynthetic pathway leading to the formation of the major plant auxin indole-3-acetic acid (IAA) (Mashiguchi et al. 2011; Stepanova et al. 2011; Won et al. 2011). Accordingly, AVG treatments of A. thaliana seedlings resulted in a reduction in IAA levels (Swarup et al. 2007; Soeno et al. 2010). In contrast, ethylene is known to stimulate auxin biosynthesis by activating the tryptophan aminotransferases mentioned above (Stepanova et al. 2005, 2007; Swarup et al. 2007). Based on auxin application experiments using preveraison grape berries and changes in the concentration of IAA levels during berry development (Inaba et al. 1976; Cawthon and Morris 1982; Zhang et al. 2003; Deytieux-Belleau et al. 2007; Böttcher et al. 2010), it has been suggested that low IAA levels are a requirement for the commencement of grape berry ripening (Böttcher et al. 2010). It is therefore possible that the AVG- and CEPA-induced effects on berry ripening observed in this study were indirectly brought about through changes in auxin levels in the fruit.
Although both ABA and AVG advanced ripening, when they were applied at particular stages of berry development, the differences in TSS accumulation (and in the other parameters measured) between the control and treated fruit were modest. This may be attributed to several factors. Over recent years, the growing seasons in the two regions used in these studies have become compressed, with the ripening of all varieties occurring earlier than usual. The causes for this phenomenon are unproven but it has been suggested that this results from reduced water inputs (including reduced rainfall) and high temperatures, probably as a result of climate change (Webb et al. 2011). Under these conditions, fruit are developing very rapidly and thus it may be difficult to further advance the timing of ripening.
The expression of ethylene biosynthesis genes is consistent with a role for ethylene throughout development
As mentioned in the Introduction (and reviewed by Böttcher and Davies 2012), the published levels and patterns of ethylene accumulation are highly variable and the measurements have been performed on detached fruit (usually including a vacuum treatment) that may result in spurious levels being detected. Some studies have reported a small rise in ethylene levels at around the time of veraison but others have not detected such an increase. Given that ethylene can inhibit ripening during the preveraison period (Hale et al. 1970; Coombe and Hale 1973; this study) and that ethylene has also been implicated in the hastening of ripening (Szyjewicz et al. 1984; Böttcher and Davies 2012; this study) it seemed important to investigate the potential for ethylene to be synthesised and perceived during berry development through measuring the expression of the genes involved. It seems that the manner of ethylene biosynthesis could change throughout berry development. ACS1 and ACO1 appear to act in concert, as they have a similar pattern of expression (even though the ratio between ACO1 and ACS1 at maximal expression levels for each is ~7 : 1), being elevated in young berries and low during ripening (Fig. 1). ACS2 and ACO2 also have similar patterns of expression, as both are expressed throughout berry development and are more highly expressed during ripening (Fig. 1). ACO2 is very highly expressed and the ratio of expression between ACO2 and ACS2 is remarkably polarised (~4800 : 1 at maximal levels for each). The low levels of ACS gene expression during ripening compared with the high level of ACO genes might indicate that ethylene expression is being controlled by the ACS enzymes during this period and that ethylene synthesis during ripening, although required, may not be high. The expression pattern of ACO3 cannot be explained at this stage. Other reports indicate that the transcript levels and enzyme activity of ACOs tend to decrease at around the time of veraison (Chervin et al. 2004; Deluc et al. 2007; Pilati et al. 2007) and that the levels of ACS transcripts and protein also decline after veraison (Deluc et al. 2007; Giribaldi et al. 2007).
Another explanation for the differing response to CEPA and AVG with time could be that the sensitivity of the ethylene biosynthesis and perception pathways to exogenous ethylene levels may vary during berry development. ACS1, ACS2 and ACO3 genes did not respond significantly to CEPA treatment (Fig. 3). However, ACO1 and ACO2 were both induced by CEPA in the earlier stages of berry development (Fig. 3) when CEPA treatment appeared to have a delaying effect on ripening (Table 2). The response to AVG also changed during berry development. The largest change was for ACS1, which was repressed by AVG treatment at 21 days before veraison but not at 6 days before veraison (Fig. 5). These results suggest that not only are there changes in the expression of these genes during ‘normal’ development but their response to exogenous ethylene-releasing (CEPA) and ethylene biosynthesis-inhibiting (AVG) agents also changes with time, perhaps through a change in the berries’ sensitivity to ethylene. The expression of the four ethylene receptors also varied during development, and the decrease in ERS1 and ETR2 expression during the preveraison stage may indicate an increase in sensitivity to ethylene during development, including the period when the response to ethylene appears to change (Fig. 2). ETR1 expression, however, does seem to increase over this period. These results are somewhat similar to those reported from microarray studies of grape berry gene expression (Deluc et al. 2007) but differ in some respects to the results of Chervin and Deluc (2010).
In contrast to the ethylene biosynthesis pathway genes, the response of the four putative receptor genes to CEPA and AVG was quite consistent. EIN4, ETR2 and ERS1 mRNA levels were increased by CEPA treatment and reduced by AVG treatment (Figs 4, 6). ETR1 was largely unresponsive to either AVG or CEPA treatments. This lack of response has been previously described for ETR1 homologues in other species, for example, in tobacco (Nicotiana tabacum L.) (Knoester et al. 1997), tomato (Solanum lycopersicum L.) (Zhou et al. 1996) and persimmon (Diospyros kaki Thunb.) (Pang et al. 2007). An increase in ethylene receptor gene transcript levels resulting from ethylene or CEPA treatment has been observed in other plants; however, at certain ethylene concentrations, the amount of receptor protein is not correlated with gene expression (Chen et al. 2007). A future study of ETR protein levels during berry development and in response to stimuli such as CEPA and AVG would be helpful in defining the molecular control of ethylene perception. The results above show that the ethylene receptors remain responsive throughout the period when AVG and CEPA were applied, and affected berry development.
In summary, the response to ethylene clearly changes during development but the modulation observed in the transcription of genes involved in ethylene biosynthesis and perception does not offer a ready explanation for these changes or the response to AVG and CEPA. The explanation may lie in the extensive interactions that occur between the various plant regulatory pathways, as changes in these pathways may affect the berries’ response to ethylene.
Acknowledgements
This work was funded by CSIRO, a partner in the Wine Innovation Cluster (WIC), and the Grape and Wine Research and Development Corporation. We would like to thank our industry collaborators, Chalk Hill Wines and Nepenthe Wines, for providing and managing vines for experimentation. Berry tissue used for the 2007–08 Shiraz developmental series was kindly supplied by Ms Lauren Hooper. ReTain was a kind gift from Valent BioSciences Australia. We also thank Dr Crista Burbidge for assistance with the preparation of the manuscript.
References
Alleweldt G, Koch R (1977) Ethylene content in ripening grape berries. Vitis 16, 263–271.Boller T, Herner RC, Kende H (1979) Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid. Planta 145, 293–303.
| Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXktFKjt7g%3D&md5=7d6f0b1d5a411608ecb6e55a07bff7a7CAS |
Böttcher C, Davies C (2012) Hormonal control of grape berry development and ripening. In ‘The biochemistry of the grape berry’. (Eds H Gerós, MM Chaves and S Delrot) pp. 194–217. (Bentham Science: Sharjah, U.A.E.)
Böttcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3–1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. Journal of Experimental Botany 61, 3615–3625.
| Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3–1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening.Crossref | GoogleScholarGoogle Scholar | 20581124PubMed |
Böttcher C, Boss PK, Davies C (2011a) Acyl substrate preferences of an IAA-amido synthetase account for variations in grape (Vitis vinifera L.) berry ripening caused by different auxinic compounds indicating the importance of auxin conjugation in plant development. Journal of Experimental Botany 62, 4267–4280.
| Acyl substrate preferences of an IAA-amido synthetase account for variations in grape (Vitis vinifera L.) berry ripening caused by different auxinic compounds indicating the importance of auxin conjugation in plant development.Crossref | GoogleScholarGoogle Scholar | 21543520PubMed |
Böttcher C, Harvey K, Forde CG, Boss PK, Davies C (2011b) Auxin treatment of pre-veraison grape (Vitis vinifera L.) berries both delays ripening and increases the synchronicity of sugar accumulation. Australian Journal of Grape and Wine Research 17, 1–8.
| Auxin treatment of pre-veraison grape (Vitis vinifera L.) berries both delays ripening and increases the synchronicity of sugar accumulation.Crossref | GoogleScholarGoogle Scholar |
Böttcher C, Boss PK, Davies C (2012) Delaying Riesling grape berry ripening with a synthetic auxin affects malic acid metabolism and sugar accumulation, and alters wine sensory characters. Functional Plant Biology 39, 745–753.
| Delaying Riesling grape berry ripening with a synthetic auxin affects malic acid metabolism and sugar accumulation, and alters wine sensory characters.Crossref | GoogleScholarGoogle Scholar |
Cawthon DL, Morris JR (1982) Relationship of seed number and maturity to berry development, fruit maturation, hormonal changes, and uneven ripening of Concord (Vitis labrusca L.) grapes. Journal of the American Society for Horticultural Science 107, 1097–1104.
Cazzonelli CI, Cavallaro AS, Botella JR (1998) Cloning and characterisation of ripening-induced ethylene biosynthetic genes from non-climacteric pineapple (Ananas comosus) fruits. Australian Journal of Plant Physiology 25, 513–518.
| Cloning and characterisation of ripening-induced ethylene biosynthetic genes from non-climacteric pineapple (Ananas comosus) fruits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXls1Kru7o%3D&md5=ea5944d1693c7ec560d9ee9ebb7d01e4CAS |
Chen JJ, Lee T, Delongchamp RR, Chen T, Tsai C-A (2007) Significance analysis of groups of genes in expression profiling studies. Bioinformatics 23, 2104–2112.
| Significance analysis of groups of genes in expression profiling studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVWqtrnP&md5=3544f399f6114a879a3ea672a275c2a0CAS | 17553853PubMed |
Chervin C, Deluc L (2010) Ethylene signalling receptors and transcription factors over the grape berry development: gene expression profiling. Vitis 49, 129–136.
Chervin C, El-Kereamy A, Roustan J-P, Latché A, Lamon J, Bouzayen M (2004) Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Science 167, 1301–1305.
| Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnslGisL4%3D&md5=20d7329de446f6c32cc75316841fef4eCAS |
Chervin C, Tira-Umphon A, Chatelet P, Jauneau A, Boss PK, Tesniere C (2009) Ethylene and other stimuli affect expression of the UDP glucose-flavonoid 3-O-glucosyltransferase in a non-climacteric fruit. Vitis 48, 11–16.
Coombe BG (1973) The regulation of set and development of the grape berry. Acta Horticulturae 34, 261–274.
Coombe BG, Hale CR (1973) The hormone content of ripening grape berries and the effects of growth substance treatments. Plant Physiology 51, 629–634.
| The hormone content of ripening grape berries and the effects of growth substance treatments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXktVWhsbw%3D&md5=188d367d3f5511fd61c6b6fc728f966eCAS | 16658384PubMed |
Davies C, Robinson SP (1996) Sugar accumulation in grape berries – cloning of two putative vacuolar invertase cDNAs and their expression in grapevine tissues. Plant Physiology 111, 275–283.
| Sugar accumulation in grape berries – cloning of two putative vacuolar invertase cDNAs and their expression in grapevine tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XivFekurk%3D&md5=8c0144e926728bb3da8f74a653a2b53fCAS | 8685267PubMed |
Davies C, Robinson SP (2000) Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress response proteins. Plant Physiology 122, 803–812.
| Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress response proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktFSqtrs%3D&md5=0cccdc420b9a69f0bf6e5dc1a7efe123CAS | 10712544PubMed |
Davies C, Boss PK, Robinson SP (1997) Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and aters the expression of developmentally regulated genes. Plant Physiology 115, 1155–1161.
Deluc L, Grimplet J, Wheatley M, Tillett R, Quilici D, Osborne C, Schooley D, Schlauch K, Cushman J, Cramer G (2007) Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics 8, 429
| Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development.Crossref | GoogleScholarGoogle Scholar | 18034876PubMed |
Deytieux C, Geny L, Lapaillerie D, Claverol S, Bonneu M, Donèche B (2007) Proteome analysis of grape skins during ripening. Journal of Experimental Botany 58, 1851–1862.
| Proteome analysis of grape skins during ripening.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsFeis7c%3D&md5=6c6022571431b4737a00509148e38b88CAS | 17426054PubMed |
Deytieux-Belleau C, Gagne S, L’Hyvernay A, Doneche B, Geny L (2007) Possible roles of both abscisic acid and indol-acetic acid in controlling grape berry ripening process. Journal International Des Sciences De La Vigne Et Du Vin 41, 141–148.
Düring H, Alleweldt G, Koch R (1978) Studies on hormonal control of ripening in berries and grape vines. Acta Horticulturae 80, 397–405.
El-Kereamy A, Chervin C, Roustan J-P, Cheynier V, Souquet J-M, Moutounet M, Raynal J, Ford C, Latché A, Pech J-C, Bouzayen M (2003) Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiologia Plantarum 119, 175–182.
| Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnvVyhsb0%3D&md5=c9d27fbe67cd61b073d9fcfe415c7d82CAS |
Frohman MA, Dush MK, Martin GR (1988) Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proceedings of the National Academy of Sciences of the United States of America 85, 8998–9002.
| Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXntVCmtQ%3D%3D&md5=174c55e47b358be77ac8003250d08394CAS | 2461560PubMed |
Fujita A, Goto-Yamamoto N, Aramski I, Hashizume K (2006) Organ-specific transcription of putative flavonol synthase genes of grapevine and effects of plant hormones and shading on flavonol biosynthesis in grape berry skins. Bioscience, Biotechnology, and Biochemistry 70, 632–638.
| Organ-specific transcription of putative flavonol synthase genes of grapevine and effects of plant hormones and shading on flavonol biosynthesis in grape berry skins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjslamt7Y%3D&md5=6318737ccc3843149c39ba8f0bf08f87CAS | 16556978PubMed |
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16, S170–S180.
Giribaldi M, Perugini I, Sauvage F-X, Schubert A (2007) Analysis of protein changes during grape berry ripening by 2-DE and MALDI-TOF. Proteomics 7, 3154–3170.
Gladstones JS (1992) ‘Viticulture and environment.’ (Winetitles: Adelaide, Australia)
Grimplet J, Deluc L, Tillett R, Wheatley M, Schlauch K, Cramer G, Cushman J (2007) Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics 8, 187
| Tissue-specific mRNA expression profiling in grape berry tissues.Crossref | GoogleScholarGoogle Scholar | 17584945PubMed |
Hale C (1968) Growth and senescence of the grape berry. Australian Journal of Agricultural Research 19, 939–945.
| Growth and senescence of the grape berry.Crossref | GoogleScholarGoogle Scholar |
Hale CR, Coombe BG (1974) Abscisic acid: an effect on the onset of rippening of grapes (Vitis vinifera L.). Royal Society of New Zealand Bulletin 12, 831–836.
Hale CR, Coombe BG, Hawker JS (1970) Effects of ethylene and 2-chloroethylphosphonic acid on the ripening of grapes. Plant Physiology 45, 620–623.
| Effects of ethylene and 2-chloroethylphosphonic acid on the ripening of grapes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3cXkslGqsLo%3D&md5=fb7d967d7bccb09736ef9c81bab6c235CAS | 16657356PubMed |
Iannetta PPM, Laarhoven L-J, Medina-Escobar N, James EK, McManus MT, Davies HV, Harren FJM (2006) Ethylene and carbon dioxide production by developing strawberries show a correlative pattern that is indicative of ripening climacteric fruit. Physiologia Plantarum 127, 247–259.
| Ethylene and carbon dioxide production by developing strawberries show a correlative pattern that is indicative of ripening climacteric fruit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtFSrsr8%3D&md5=1e3f02cbd4223a8c05ffc4f8878a9849CAS |
Inaba A, Ishida M, Sobajima Y (1976) Changes in endogenous hormone concentrations during berry development in relation to ripening of Delaware grapes. Journal of the Japanese Society for Horticultural Science 45, 245–252.
| Changes in endogenous hormone concentrations during berry development in relation to ripening of Delaware grapes.Crossref | GoogleScholarGoogle Scholar |
Katz E, Lagunes P, Riov J, Weiss D, Goldschmidt E (2004) Molecular and physiological evidence suggests the existence of a system II-like pathway of ethylene production in non-climacteric Citrus fruit. Planta 219, 243–252.
| Molecular and physiological evidence suggests the existence of a system II-like pathway of ethylene production in non-climacteric Citrus fruit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksVSgsLw%3D&md5=baee3650222674a8cc55ff696835eb91CAS | 15014996PubMed |
Kevany BM, Tieman DM, Taylor MG, Cin VD, Klee HJ (2007) Ethylene receptor degradation controls the timing of ripening in tomato fruit. The Plant Journal 51, 458–467.
| Ethylene receptor degradation controls the timing of ripening in tomato fruit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVKju7o%3D&md5=2163b5dd74dabe01cc9c3501930ed43eCAS | 17655616PubMed |
Kevany BM, Taylor MG, Klee HJ (2008) Fruit-specific suppression of the ethylene receptor LeETR4 results in early-ripening tomato fruit. Plant Biotechnology Journal 6, 295–300.
| Fruit-specific suppression of the ethylene receptor LeETR4 results in early-ripening tomato fruit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksl2is7Y%3D&md5=76e0cd5ada01276eb3869e2e76818b7eCAS | 18086233PubMed |
Knoester M, Hennig J, van Loon L, Bol J, Linthorst J (1997) Isolation and characterization of a tobacco cDNA encoding an ETR1 homolog (accession no. AF022727). Plant Physiology 115, 1731
Lin Z, Zhong S, Grierson D (2009) Recent advances in ethylene research. Journal of Experimental Botany 60, 3311–3336.
| Recent advances in ethylene research.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVWitrzJ&md5=15f32a9110a0815a8de5040d51e3fb19CAS | 19567479PubMed |
Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, McSteen P, Zhao Y, Hayashi K-i, Kamiya Y, Kasahara H (2011) The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 108, 18512–18517.
| The main auxin biosynthesis pathway in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFymurvN&md5=8dbc6d34327153aaa06599f676515e9dCAS | 22025724PubMed |
Pang JH, Ma B, Sun H-J, Ortiz GI, Imanishi S, Sugaya S, Gemma H, Ezura H (2007) Identification and characterization of ethylene receptor homologs expressed during fruit development and ripening in persimmon (Diospyros kaki Thunb.). Postharvest Biology and Technology 44, 195–203.
| Identification and characterization of ethylene receptor homologs expressed during fruit development and ripening in persimmon (Diospyros kaki Thunb.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjvFOls7g%3D&md5=b014a9b6e691d335427b2a3b7ef28e03CAS |
Pierik R, Sasidharan R, Voesenek LACJ (2007) Growth control by ethylene: adjusting phenotypes to the environment. Journal of Plant Growth Regulation 26, 188–200.
| Growth control by ethylene: adjusting phenotypes to the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1yks7s%3D&md5=2a29943ef827d12906b85a4b5c0fbed0CAS |
Pilati S, Perazzolli M, Malossini A, Cestaro A, Dematte L, Fontana P, Dal Ri A, Viola R, Velasco R, Moser C (2007) Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at veraison. BMC Genomics 8, 428
| Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at veraison.Crossref | GoogleScholarGoogle Scholar | 18034875PubMed |
Roubelakis-Angelakis KA, Kliewer WM (1986) Effects of exogenous factors on phenylalanine ammonia-lyase activity and accumulation of anthocyanins and total phenolics in grape berries. American Journal of Enology and Viticulture 37, 275–280.
Sisler EC, Serek M (2003) Compounds interacting with the ethylene receptor in plants. Plant Biology 5, 473–480.
| Compounds interacting with the ethylene receptor in plants.Crossref | GoogleScholarGoogle Scholar |
Soeno K, Goda H, Ishii T, Ogura T, Tachikawa T, Sasaki E, Yoshida S, Fujioka S, Asami T, Shimada Y (2010) Auxin biosynthesis inhibitors, identified by a genomics-based approach, provide insights into auxin biosynthesis. Plant & Cell Physiology 51, 524–536.
| Auxin biosynthesis inhibitors, identified by a genomics-based approach, provide insights into auxin biosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXks1Khurc%3D&md5=13ae07f7f5cee3cf76edbce326371966CAS |
Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. The Plant Cell 17, 2230–2242.
| A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpsFGjsL8%3D&md5=3134253a2100e3746b5a5fea5db11013CAS | 15980261PubMed |
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. The Plant Cell 19, 2169–2185.
| Multilevel interactions between ethylene and auxin in Arabidopsis roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtValur%2FL&md5=64a169ed7880a74e517c9a8a99687693CAS | 17630276PubMed |
Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM (2011) The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. The Plant Cell 23, 3961–3973.
| The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvFOktw%3D%3D&md5=bd1d58a94f32d5657474a69d9d979e5fCAS | 22108406PubMed |
Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. The Plant Cell 19, 2186–2196.
| Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtValur%2FE&md5=35edd345f8ab935f9183e40f9f9ea6e6CAS | 17630275PubMed |
Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR (2006) Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiology 140, 150–158.
| Grapes on steroids. Brassinosteroids are involved in grape berry ripening.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVCgsbw%3D&md5=19cf38e44aad45fe3f5429c97b8852c8CAS | 16361521PubMed |
Szyjewicz E, Rosner N, Kliewer WM (1984) Ethephon ((2-chloroethyl)phosphonic acid, Ethrel, CEPA) in viticulture – a review. American Journal of Enology and Viticulture 35, 117–123.
Terrier N, Glissant D, Grimplet J, Barrieu F, Abbal P, Couture C, Ageorges A, Atanassova R, Léon C, Renaudin J-P, Dédaldéchamp F, Romieu C, Delrot S, Hamdi S (2005) Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta 222, 832–847.
| Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1emsrzL&md5=0a952d29fbc83baf8433c68b3ffbfde1CAS | 16151847PubMed |
Theologis A (1992) One rotten apple spoils the whole bushel: the role of ethylene in fruit ripening. Cell 70, 181–184.
| One rotten apple spoils the whole bushel: the role of ethylene in fruit ripening.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XltVyjtLs%3D&md5=815a0e7b930a86e6cbe56a87ee6b65c4CAS | 1638627PubMed |
Tira-Umphon A, Roustan J-P, Chervin C (2007) The stimulation by ethylene of the UDP glucose-flavonoid 3-O-glucosyltransferase (UFGT) in grape tissues is independent from the MybA transcription factors. Vitis 46, 210–211.
Trainotti L, Pavanello A, Casadoro G (2005) Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? Journal of Experimental Botany 56, 2037–2046.
| Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmt1GmsrY%3D&md5=ce9a81cc813b3d3563de4a47b7565410CAS | 15955790PubMed |
Wang H, Huang H, Huang X (2007) Differential effects of abscisic acid and ethylene on the fruit maturation of Litchi chinensis Sonn. Plant Growth Regulation 52, 189–198.
| Differential effects of abscisic acid and ethylene on the fruit maturation of Litchi chinensis Sonn.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntVOmu7k%3D&md5=494bf91716131caac9b1cac3354a61acCAS |
Waters DLE, Holton TA, Ablett EM, Lee LS, Henry RJ (2005) cDNA microarray analysis of developing grape (Vitis vinifera cv. Shiraz) berry skin. Functional & Integrative Genomics 5, 40–58.
| cDNA microarray analysis of developing grape (Vitis vinifera cv. Shiraz) berry skin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVOqs7%2FF&md5=448c101863888144deb61a863e71194cCAS |
Weaver RJ (1962) The effect of benzothiazole-2-oxyacetic acid on maturation of seeded varieties of grapes. American Journal of Enology and Viticulture 13, 141–149.
Weaver RJ, Singh IS (1978) Occurrence of endogenous ethylene and effect of plant regulators on ethylene production in the grapevine. American Journal of Enology and Viticulture 29, 282–285.
Webb LB, Whetton PH, Barlow EWR (2011) Observed trends in winegrape maturity in Australia. Global Change Biology 17, 2707–2719.
| Observed trends in winegrape maturity in Australia.Crossref | GoogleScholarGoogle Scholar |
Wheeler S, Loveys B, Ford C, Davies C (2009) The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Australian Journal of Grape and Wine Research 15, 195–204.
| The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlOrsr7L&md5=d1a8f214f10c8a7019e2d69ba82852d8CAS |
Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 108, 18518–18523.
| Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFymu7zK&md5=b9d3a9e5606247f01dbeb74962bf77b0CAS | 22025721PubMed |
Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LA, Theologis A (2003) Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. The Journal of Biological Chemistry 278, 49102–49112.
| Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptlGrs70%3D&md5=892a32d37e1bf848c5286e2b28a3e582CAS | 12968022PubMed |
Yu Y-B, Adams DO, Yang SF (1979) 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Archives of Biochemistry and Biophysics 198, 280–286.
| 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXltl2gtg%3D%3D&md5=83597b79a33ee6ba846b6231d60447a1CAS | 507845PubMed |
Zhang XR, Luo GG, Wang RH, Wang J, Himelrick DG (2003) Growth and developmental responses of seeded and seedless grape berries to shoot girdling. Journal of the American Society for Horticultural Science 128, 316–323.
Zhou DB, Kalaitzis P, Mattoo AK, Tucker ML (1996) The mRNA for an ETR1 homologue in tomato is constitutively expressed in vegetative and reproductive tissues. Plant Molecular Biology 30, 1331–1338.
| The mRNA for an ETR1 homologue in tomato is constitutively expressed in vegetative and reproductive tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xks1Kisb4%3D&md5=4a924fac7945338938fa865fb7afb702CAS |





