A survey of genes involved in Arachis stenosperma resistance to Meloidogyne arenaria race 1
Carolina V. Morgante A E , Ana C.M. Brasileiro B , Philip A. Roberts C , Larissa A. Guimaraes B , Ana C.G. Araujo B , Leonardo N. Fonseca B , Soraya C.M. Leal-Bertioli B , David J. Bertioli D and Patricia M. Guimaraes BA Embrapa Semiárido, BR 428, Km 152, CP 23, 56302-970, Petrolina, PE, Brazil.
B Embrapa Recursos Genéticos e Biotecnologia, PqEB – Av W5 Norte, CP 02372, 70770-917, Brasília, DF, Brazil.
C University of California, Nematology Department, 2251 Spieth Hall Riverside, CA 92521, USA.
D Universidade de Brasília, Departamento de Genética e Morfologia, Campus Universitario Darcy Ribeiro, 70910-900, Brasília, DF, Brazil.
E Corresponding author. Email: carolina.morgante@embrapa.br
This paper originates from a presentation at the ‘VI International Conference on Legume Genetics and Genomics (ICLGG)’ Hyderabad, India, 2–7 October 2012.
Functional Plant Biology 40(12) 1298-1309 https://doi.org/10.1071/FP13096
Submitted: 22 April 2013 Accepted: 11 July 2013 Published: 19 August 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Root-knot nematodes constitute a constraint for important crops, including peanut (Arachis hypogaea L.). Resistance to Meloidogyne arenaria has been identified in the peanut wild relative Arachis stenosperma Krapov. & W. C. Greg., in which the induction of feeding sites by the nematode was inhibited by an early hypersensitive response (HR). Here, the transcription expression profiles of 19 genes selected from Arachis species were analysed using quantitative reverse transcription–polymerase chain reaction (qRT-PCR), during the early phases of an A. stenosperma–M. arenaria interaction. Sixteen genes were significantly differentially expressed in infected and non-infected roots, in at least one of the time points analysed: 3, 6, and 9 days after inoculation. These genes are involved in the HR and production of secondary metabolites related to pathogen defence. Seven genes encoding a resistance protein MG13, a helix-loop helix protein, an ubiquitin protein ligase, a patatin-like protein, a catalase, a DUF538 protein, and a resveratrol synthase, were differentially expressed in all time points analysed. Transcripts of two genes had their spatial and temporal distributions analysed by in situ hybridisation that validated qRT-PCR data. The identification of candidate resistance genes involved in wild peanut resistance to Meloidogyne can provide additional resources for peanut breeding and transgenic approaches.
Additional keywords: hypersensitive response, in situ hybridisation, peanut, qRT-PCR, root-knot nematode, wild Arachis.
Introduction
Root-knot nematodes (Meloidogyne spp.) are the most frequently observed biotrophic nematode parasites in plants, with a broad host range of more than 2000 species, including many crops (Agrios 2005). These nematodes are obligate endoparasites that occur in tropical and temperate regions, and cause significant economic losses in peanut production areas worldwide (Holbrook et al. 2000). The most widely used strategy to control root-knot nematodes is the use of nematicides, which are often highly toxic and have been progressively restricted or banned due to environmental and human health concerns (Collange et al. 2011). The use of resistant cultivars is one of the most promising practices to reduce crop damage in an integrated pest management approach. Indeed, host resistance against nematodes has been identified in many crop species, including soybean (Davis et al. 1998), cowpea (Olowe 2009), coffee (Albuquerque et al. 2010), and also peanut, Arachis hypogaea L. (Castillo et al. 1973). Resistance against M. arenaria race 1, the most damaging nematode for peanut, has been observed in the wild species Arachis cardenasii Krapov. & W. C. Greg., Arachis batizocoi Krapov. & W. C. Greg. and A. stenosperma Krapov. & W. C. Greg., as shown by the decrease in the number of females and the delay in the pathogen life cycle (Nelson et al. 1990; Choi et al. 1999; Bendezu and Starr 2003). Further, two peanut cultivars, COAN and Nematan, resistant to this nematode were developed by introgressing a dominant gene from A. cardenasii (Nagy et al. 2010). It is important that new sources of Arachis resistance to M. arenaria are identified in Arachis, in order to decrease the risk of resistance breakdown due to the emergence of new virulent nematode populations.
Nematode infection initiates complex changes in plant gene expression (Williamson and Gleason 2003; Davis and Mitchum 2005), starting with infective second-stage nematode juveniles (J2) entering host roots and migrating toward the vascular cylinder in search of suitable cells for initial feeding sites establishment. J2 then initiate localised reorganisation of host cell morphology and physiology, resulting in the formation of specialised feeding cells (Jones 1981). In the case of Meloidogyne spp., the feeding cells enlarge and become multinucleated through divisions in absence of cytokinesis, and are called ‘giant cells’ (Huang 1985). The understanding of cell and molecular mechanisms of host–pathogen interactions may provide additional resources for developing new forms of durable plant defences (Williamson and Kumar 2006).
A genome overview of gene expression in Arabidopsis infected with M. incognita by microarray analysis showed that approximately 15% of the genes displayed significant differential expression between non-infected roots and nematode galls at different stages of the infection, evidence of the complexity of nematode feeding-site development (Jammes et al. 2005). Likewise, altered expression of numerous genes during the interaction with Meloidogyne spp. in soybean roots has also been demonstrated (Ibrahim et al. 2011; de Sá et al. 2012).
So far, the mechanisms of resistance of wild Arachis to root-knot nematodes have been characterised in detail only in A. stenosperma, which shows typical symptoms of an early hypersensitive response (HR). In this species, the induction of nematode feeding sites was associated with a necrotic-like response, thereby hindering nematode development, root-galling and egg mass formation (Proite et al. 2008).
Nematode infection triggers pathways related to defence response, including HR genes, as well as those involved in the redesign of root morphology to form galls and giant cells for feeding (Ibrahim et al. 2011). As a vast suite of genes are induced in defence response against root-knot nematodes and other pests, a specific recognition of the pathogen by the plant may lead to resistance through HR, accompanied by rapid cell death in and around the infection site, thus, effectively containing pathogens at their site of entry (Lam 2004). Early HR against different species of Meloidogyne has been described in several plants, such as cotton (Mota et al. 2012), wild peanut (Proite et al. 2008), Mi-1-mediated resistance in tomato (Vos et al. 1998), Mex-1-mediated resistance in coffee (Anthony et al. 2005), and Me3-mediated resistance in pepper (Pegard et al. 2005). In addition, complex networks of genes that interfere in metabolic pathways, cell-cycle progression and water transport are among those whose expression is increased in the feeding cells as well as in the resistance response (Williamson and Gleason 2003), which demonstrates the enormous challenge to select and identify reliable candidate genes for further validation and application to broadening plant resistance to root-knot nematode.
In peanut and its wild relatives, only a few studies analysing transcriptome profiles have dealt with plant–nematode interactions. In cultivated peanut infected with M. arenaria, Tirumalaraju et al. (2011) showed several genes were differentially expressed in resistant and susceptible cultivars. Likewise, the analysis of expressed sequence tags (ESTs) derived from wild A. stenosperma revealed a number of differentially expressed genes between inoculated and control plants; these being related to hormonal balance, defence and alleviation of oxidative stress triggered by HR (Proite et al. 2007; Guimarães et al. 2010).
In the present study, the expression profiles of 19 nematode resistance candidate genes selected either from our wild Arachis EST databanks or public databases were analysed using quantitative reverse transcription–polymerase chain reaction (qRT-PCR) and in situ hybridisation. Analyses focussed on the early phases of A. stenosperma–M. arenaria interaction, with the aim to identify those genes potentially involved in triggering the HR response and inhibition of giant cell formation.
Materials and methods
Plant material and nematode challenge
Seeds of Arachis stenosperma Krapov. & W. C. Greg. (accession V10309), resistant to Meloidogyne arenaria race 1, and A. hypogaea L. cv. Florunner, a susceptible cultivar, were treated with Ethrel (Bayer Crop Science, Research Triangle Park, NC, USA) 0.1% (v/v) for 12 h, and planted singly in 600 cm3 pots containing steam-sterilised soil. Plants were maintained under greenhouse conditions at the University of California, Riverside, USA, at 28−35°C air temperature, and watered daily. Eggs of M. arenaria race 1 were cultured on susceptible tomato cv. UC82 host plants, extracted from roots using 10% (v/v) bleach solution (Hussey and Barker 1973) and hatched at 28°C in an incubator. Approximately 20 000 juveniles (J2) resuspended in fresh deionised water were pipetted onto soil depressions around each of the 4-week-old plants of A. stenosperma and A. hypogaea. Control individuals received mock inoculum consisting of deionised water only. Plants were arranged randomly on a bench, and roots collected at 0, 3, 6, and 9 days after inoculation (DAI). Collected roots were either immediately frozen in liquid nitrogen and stored at −80°C for RNA extraction or immersed in a cacodylate solution containing glutaraldehyde and paraformaldehyde for in situ hybridisation procedures, as detailed below.
RNA extraction and cDNA synthesis
Total RNA was extracted from roots (250 mg) of A. stenosperma individual plants, following the lithium chloride modified protocol described by Morgante et al. (2011), and purified with Invisorb Plant RNA Mini Kit (Invitek, Berlin, Germany). Total RNA integrity was checked by 1% (v/v) gel electrophoresis and its concentration was quantified on NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Equal amounts of total RNA per collecting point (0, 3, 6, and 9 DAI) were pooled from three plants, in two independent biological replicates. A total of 2 µg of each RNA pool was treated with 2 U of DNase (Fermentas, St Leon-Rot, Germany) to eliminate genomic DNA, and reverse transcribed using the Super Script II enzyme and oligo(dT)20 primer (Invitrogen, Carlsbad, CA, USA), according to manufacturer’s instructions. DNAse treatment and cDNA synthesis were performed in subsequent steps, in the same tube, to avoid nucleic acid loss during precipitation and washing steps. cDNA samples were diluted (1 : 100) and stored at −20°C.
Elimination of DNA contamination in cDNA samples was further checked by real-time PCR using primers from Arachis magna Krapov. & W. C. Greg. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) coding gene (GAPDH-F 5′-CAACAACGGAGACATCAACG-3′ and GAPDH-R 5′-ATCACTGCCACCCAGAAAAC-3′; Morgante et al. 2011) that flank an intron region in Arachis spp.
Candidate gene selection
Nineteen candidate genes were selected for transcriptional profile analysis in plants of A. stenosperma challenged with nematodes by using qRT-PCR (Table 1). Twelve genes were chosen based on our previous work (Proite et al. 2008; Guimarães et al. 2010) and our wild Arachis transcripts database. The remaining genes were selected considering their role in root-knot resistance in plants (Jammes et al. 2005; Tirumalaraju et al. 2011; Kyndt et al. 2012) and A. stenosperma homologous sequences identified using BLAST tools (Altschul et al. 1990).
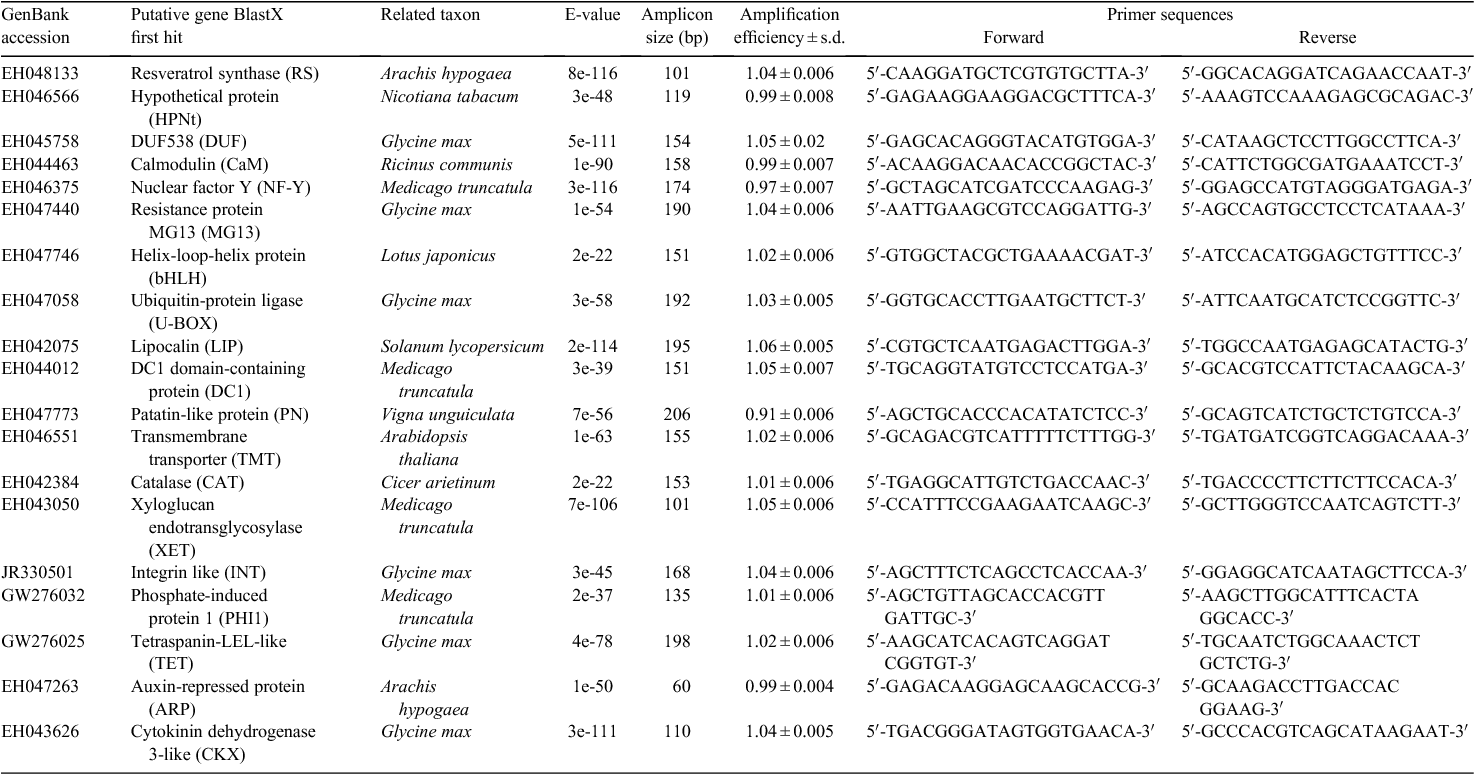
|
For each of the 19 candidate genes, primers were designed based on the contig alignments, using Primer 3 Plus software (Untergasser et al. 2007), with lengths between 19 and 22 nucleotides, melting temperature between 55 and 62°C, G/C content between 45 and 55%, and PCR products ranging from 100 to 210 bp. Gene-specificity of primers was confirmed using BLAST searches on the A. stenosperma transcripts database resources (Expressed Sequence Tags (EST) and Transcriptome Shotgun Assembly (TSA)) from the National Centre for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/, accessed 10 May 2012). Amplicon length was checked by real-time PCR using an equimolar pool of all cDNA samples as template. Primer sequences, length of products, and source of sequences are listed in Table 1.
qRT-PCR analyses
qRT-PCR assays were performed using 5 µL of Platinum SYBR Green qPCR Super Mix-UDG w/ROX kit (Invitrogen) in a 10 µL final volume reaction containing 2 µL of diluted cDNA and 0.2 µM of each primer. GAPDH and ribosomal 60S coding genes were used as reference for data normalisation (Morgante et al. 2011). Reactions were conducted in three technical replicates for each sample in 96-well plates on the 7300 Real Time PCR System (Applied Biosystems, Foster City, CA, USA). The program applied was: 2 min at 50°C; 10 min at 95°C, followed by 40 cycles of 95°C (15 s) and 60°C (1 min). Dissociation curve analysis of amplification products was performed for each reaction.
Primer efficiency and optimal cycle of quantification (Cq) values were estimated using the online real-time PCR Miner tool (Zhao and Fernald 2005). Average Cq values were normalised to the two reference genes (AsGAPDH and As60S), and expression ratios of mRNA transcripts at 3, 6, and 9 DAI, relative to day 0, were calculated and statistically tested using REST 2009 ver. 2.0.13 software (Pfaffl et al. 2002). Gene expression values were further represented in a heat map using GENE-E tool (http://www.broadinstitute.org/cancer/software/GENE-E/, accessed 13 January 2013).
In situ hybridisation
Fixed roots were gradually dehydrated in a graded series of ethanol solutions (70, 90, 96, and 100%) and slowly embedded in butyl-methyl methacrylate. Semi-thin sections with 2.5–3.5 µm of thickness were mounted on histological slides and resin removed from the sections by repeated washes with acetone. One slide of each material was stained with acridine orange in order to confirm RNA preservation of processed samples.
The mRNA sequences of A. stenosperma genes coding an auxin-repressed protein (AsARP) and a cytokinin dehydrogenase 3-like (AsCKX) were amplified and cloned into pGEM-T Easy Vector (Promega, Madison, WI, USA). The plasmids were linearised by digestion with Sac I or Sac II restriction enzymes and used as probes, after labelling with the Digoxigenin RNA in vitro transcription labelling kit (Roche, Indianapolis, IN, USA), according to the manufacturer’s instructions. Root sections were pre-treated and hybridised with 2 ng of the labelled probe in 1 μL of hybridisation solution in a humid chamber at 42°C for 12 h, as described by Tucker et al. (2003). Hybridisation sites were immunocytochemically detected using an anti-digoxigenin antibody conjugated to alkaline phosphatase (Roche), followed by incubation with NBT/BCIP buffer (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate) for colorimetric detection of the phosphatase activity. Slides were mounted in glycerol and analysed under an epifluorescent microscope (Carl Zeiss, Zeiss, Germany).
Results
In order to identify and characterise genes involved in the incompatible interaction between A. stenosperma and M. arenaria race 1, the transcriptional profile of 19 candidate genes potentially involved in the resistance response were analysed by qRT-PCR, and, in some cases, also by in situ hybridisation, during the early stages of the infection.
Nematode infection and RNA extraction
Roots from the resistant A. stenosperma were inoculated with J2 stage of M. arenaria race 1 and the onset of disease symptoms was compared with non-infected roots. Susceptible A. hypogaea cv. Florunner plants, used as positive controls for the nematode infection, showed swollen roots corresponding to nematode feeding sites around 9 DAI, and numerous root-galls at 30 DAI (data not shown). As expected, no infection symptoms were visible in the root systems of inoculated A. stenosperma. This is in line with our previous work where no tissue hypertrophy or cell hyperplasia was observed after nematode inoculation in the resistant species A. stenosperma (Proite et al. 2008).
Total purified RNA from all root samples showed high quality and the formed pools were reverse transcribed for qRT-PCR analysis. The absence of genomic DNA was confirmed by using GAPDH primers, as the expected amplicons have 190 and 340 bp from cDNA and genomic templates, respectively, allowing the distinction between PCR products. The different sizes are apparently due to the presence of an intron flanked by GAPDH primers. These primers, originally designed for A. magna (Morgante at al. 2011), also generated distinct products amplified from genomic DNA and cDNA templates in other Arachis species (data not shown), suggesting their broader application for the genus. RT-PCR provides a very sensitive method for detecting very small amounts of DNA contamination in RNA or cDNA samples and is a crucial step to assure sample purity and quality and to avoid false-positive results in qRT-PCR assays (Taylor et al. 2010).
Gene expression profiles
From the 19 selected genes related to plant response against root-knot nematodes (Table 1), 17 showed homology in BlastX to A. stenosperma sequences in transcripts database resources, for which primers were designed. Two genes, AsPHI1 and AsTET, had no similarities identified in the A. stenosperma database and therefore previously described A. hypogaea primers were used (Tirumalaraju et al. 2011). The transferability of these primers among Arachis species was supported by their similarity to other wild species transcript sequences, including Arachis ipaënsis Krapov. & W. C. Greg. and Arachis duranensis Krapov. & W. C. Greg.
The PCR Miner algorithm was used to evaluate the amplification efficiency from each fluorescence raw data as an input (Zhao and Fernald 2005). This statistical algorithm, which does not require the establishment of a standard curve, proved to be fast and simple to use, as previously suggested (Marum et al. 2012). All primer pairs showed high efficiency, ranging from 0.91 to 1.06 (Table 1), and were used to adjust Cq values in subsequent analyses. The melt curve analysis supported the specificity of amplification of all transcripts as a single amplicon and no primer dimers were observed.
AsGAPDH and As60S were chosen as reference genes for qRT-PCR as they were established as the most stably expressed genes in roots of A. stenosperma subjected to biotic stress (Morgante et al. 2011). Comparison of absolute expression (Cq) values in all samples resulted in very similar expression levels, with AsGAPDH median= 23.08, quartiles 22.14–23.74, and As60S median = 23.28, quartiles 22.68–23.96. This indicated their stability along the assay time-course in both challenged and control A. stenosperma plants. These data corroborate our previous conclusions that these two genes are adequate for normalisation (Morgante et al. 2011), and adhere to the recommended use of more than one reference gene in qPCR analysis (Taylor et al. 2010).
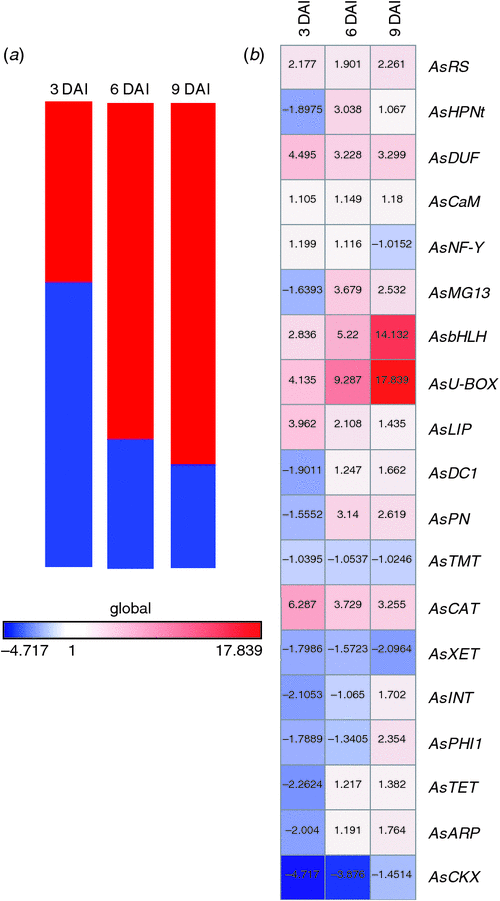
Relative expression analysis of the 19 candidate genes within fold changing from –4.72 to 17.84 and their corresponding profiles are represented as a heat map (Fig. 1). The global expression pattern indicated a downregulation for the majority (58%) of the analysed genes at 3 DAI. However, throughout the assay, an increase in expression was observed, and 79% of the genes were upregulated at 9 DAI. These results agree with the expression profiles reported during root-knot nematode infection in Arachis (Guimarães et al. 2010; Tirumalaraju et al. 2011) and other plants, such as rice (Kyndt et al. 2012), Arabidopsis (Jammes et al. 2005; Barcala et al. 2010), tomato (Portillo et al. 2013) and cowpea (Das et al. 2010). These studies reported gene repression or constant expression at early stages of nematode–plant interaction, followed by a trend towards an increasing number of upregulated genes in both compatible and incompatible interactions. However, only a few differentially expressed genes involved in defence/stress responses were shared between both interactions. In our study, the selected 19 genes related to defence/stress showed modulation along the time points studied, which might play an important role in the defence response during incompatible M. arenaria–A. stenosperma interaction, leading to the HR-mediated cell death process.
|
Expression analysis of candidate genes
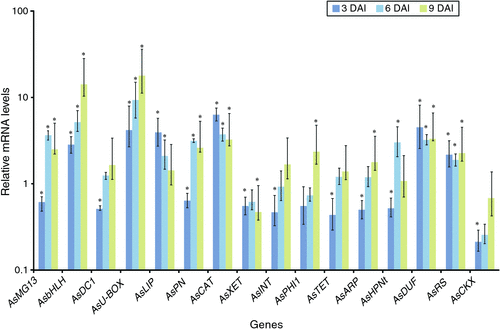
Of the 19 genes analyse, 16 showed significant differential expression in A. stenosperma roots infected with M. arenaria race 1 in at least one of the time points, seven genes being differentially expressed in all three time points analysed (Fig. 2). With the exception of AsPHI1, all genes showed significant differential expression at 3 DAI, suggesting an extensive transcriptional regulation in the early stages of nematode infection. At 6 and 9 DAI, almost half of the genes showed significant diverse expression profiles.
|
An upregulation of ~2-fold at all time points was observed for AsRS2, AsbHLH, AsU-BOX, AsDUF, and AsCAT in inoculated plants, relative to day 0 (Figs 1, 2). The most upregulated genes were those encoding bHLH and U-BOX, with maximum fold values of 14.13- and 17.84-, respectively, at 9 DAI (Fig. 1). The most downregulated gene was AsCKX, showing lower values at early stages of nematode infection (3 and 6 DAI).
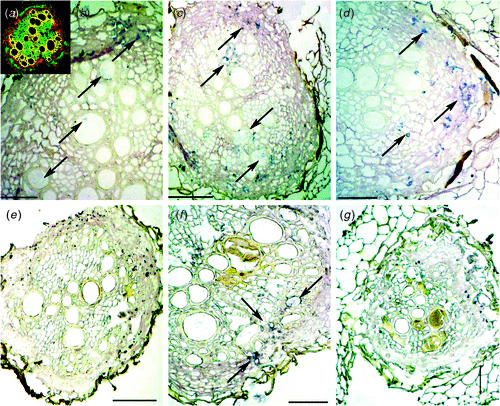
AsARP and AsCKX sequences were further used as probes for in situ hybridisation to determine the spatial distribution of its transcripts in the cells of infected and control roots during initial phases of the nematode interaction. The presence of RNA in different cells as indicated by the redish/orangish colors (Fig. 3a) was confirmed in the acridine stained sections of all samples. AsARP transcripts were detected in cortical and xylem cells of both infected and non-infected A. stenosperma roots, collected at 3, 6 and 9 DAI (Fig. 3b–d). We noted that hybridisation signals were clearly stronger and more dispersed in inoculated than control roots in these same tissues. No signals could be detected in epidermal or phloem cells. When AsCKX probes were used for in situ hybridisation analysis in A. stenosperma control roots, weak signals of AsCKX transcripts could be detected with the anti-sense probe, whilst the sense probe lacked signal. On the other hand, in inoculated A. stenosperma roots probe signal was weaker than in the control (Fig. 3e–g), confirming the low expression of this gene as indicated by qRT-PCR.
|
Discussion
Worldwide, M. arenaria race 1 is the most important nematode constraint to peanut that shows limited resistance to this parasite, with most cultivars (e.g. COAN and Nematan) relying on a single resistance source from A. cardenasii. In previous studies, Proite et al. (2008) showed that the resistance harboured by the wild peanut relative A. stenosperma was due to a HR-mediated cell death process, which is characterised by the rapid development of cell death immediately surrounding infection sites (Morel and Dangl 1997).
In order to identify and characterise genes involved in this incompatible interaction, the transcriptional profile of 19 candidate genes potentially involved in the resistance response were analysed in the early stages of the infection.
The 16 differentially expressed genes during early stages of an A. stenosperma–M. arenaria race 1 interaction identified were placed into five general groups, on the basis of their GO annotation (http://www.blast2go.com/b2ghome, accessed 11 July 2012), and role in plant-pathogen interaction: resistance (R) genes, transcriptional regulation, pathogen defence response, membrane/cell wall components, and hormonal balance. In those groups we found genes involved in HR and production of secondary metabolites related to pathogen defence.
R genes
Hypersensitive response can be triggered by a wide variety of pathogens and occurs within a few hours following pathogen contact (Meyers et al. 2005). In some cases this response is conditioned by the presence in the pathogen of an avirulence (avr) gene, the product of which is recognised by a plant possessing the corresponding R gene. This mechanism was described by Flor (1946) as the ‘gene-for-gene’ hypothesis. In other cases, when the Guard Model (Dangl and Jones 2001) is applied, pathogen effectors (pathogen-secreted proteins that manipulate host cell functions), are indirectly perceived by R proteins, which trigger disease resistance in the host. This model explains how multiple effectors could be recognised by a single R protein, enabling a small number of R genes to target a broad diversity of pathogens.
In the present study, an A. stenosperma homolog of soybean MG13 (AsMG13) was upregulated at 6 and 9 DAI in roots of infected plants. This gene was first identified in cDNA libraries of soybean roots infected with the cyst nematode Heterodera glycines as containing conserved R gene domains, belonging to the NBS-LRR family (Graham et al. 2000). Such plant disease R genes mediate specific pathogen recognition, often leading to successful immune response. Downstream responses of R genes include ion fluxes, oxidative burst, rapid release of reactive oxygen species (ROS), transcriptional reprogramming and, in many cases, HR causing cell death at the infection site (Tornero et al. 2002). Several sedentary nematode R genes (Mi-1, Hero A, Gpa2 and Gro1-4) resemble other plant R genes, falling into the NBS-LRR class (Williamson and Kumar 2006). Considering the compatible timing of the occurrence of the resistance-mediated by HR in A. stenosperma (8 DAI) (Proite et al. 2008) and the herein transcription profile of AsMG13, we hypothesise that this gene is involved in pathogen recognition that triggers the resistance response to M. arenaria.
Transcriptional regulation
Following the pathogen recognition step by R genes, signal transduction pathways are triggered, often leading to the induction of defence-related genes. Some of these TFs are specifically induced by a plant–pathogen interaction; however, abiotic factors and developmental events can also be a trigger (Morel and Dangl 1997).
Basic helix-loop-helix (bHLH) proteins constitute one of the largest TFs families in Arabidopsis (Heim et al. 2003). In this study, the significant upregulation of an A. stenosperma homolog (AsbHLH) in infected roots corroborates previous macroarray data (Guimarães et al. 2010). Some of these bHLH TFs form clusters regulating specific jasmonic acid (JA)-dependent responses, which modulate processes including defence against pathogens (Fernandez-Calvo et al. 2011). JA, a stress-induced hormone, is involved in defence against insects, pathogens and abiotic stresses, and can also induce the production of secondary metabolites, including alkaloids, anthocyanins, and terpenoid compounds (Niu et al. 2011). The increase in ROS levels in the cells and the induction of the synthesis of secondary metabolites like phythoalexins cause cell death (Chang et al. 2011). We suggest that the upregulation of AsbHLH during A. stenosperma response to M. arenaria infection induces the production of JA and therefore contributes to the HR response. Another TF analysed here, AsDC1, previously reported to be induced by cell wall microbe components (Shinya et al. 2007), showed an expression profile that did not correlate to the development of the defence response.
Pathogen defence response
One of the outcomes of the defence related intracellular signalling is the activation of defence genes. Seven genes involved in the synthesis of anti-microbial compounds and pathogenesis-related (PR) proteins were found to be differentially regulated in this study, as discussed below.
Patatins are glycoproteins with a non-specific lipid acyl hydrolase activity that can initiate the synthesis of fatty-acid-derived defence signals against stress (Wang 2004; Yang et al. 2007). The upregulation of patatin-like genes has been associated to defence and triggering of cell death and HR upon infection of a diverse range of pathogens (Dhondt et al. 2000, Cacas et al. 2009; Orlowska et al. 2012), including M. arenaria race 1 in A. hypogaea roots (Tirumalaraju et al. 2011). Likewise, we found upregulation of AsPN expression in roots infected with M. arenaria at 6 and 9 DAI, leading us to consider its association in the promotion of the HR response, through JA production, as previously described in Arabidopsis (Yang et al. 2007).
Another protein related to defence is resveratrol synthase (RS), the last of the four enzymes that participate in the resveratrol biosynthesis, which can be induced by different biotic and abiotic stresses (Chang et al. 2011). Indeed, the accumulation of RS and its mRNA was observed in peanut in response to treatment with stress hormone, yeast extract, ultraviolet light, and after wounding (Chung et al. 2003), fungal chitin treatment (Yang et al. 2010), and infection with Aspergillus spp. (Sobolev 2008). Recently, it was shown in Vitis vinifera that resveratrol, in addition to its classical role as an antimicrobial phytoalexin, is an important regulator for initiation of HR-related cell death (Chang et al. 2011). In the present study, a variant homolog of A. stenosperma RS gene AsRS1, previously analysed using macroarray by Guimarães et al. (2010), named AsRS2, showed strong upregulation in the three time points analysed, coinciding with the occurrence of HR in A. stenosperma. Therefore, we suggest that AsRS2 is involved in the HR response of A. stenosperma to M. arenaria and is one of our premium candidates for overexpression in transgenic peanut to enhance nematode resistance.
Several transcriptome studies have demonstrated the upregulation of PHI1 (Phosphate-induced protein 1 gene) in resistant plants in response to pathogen attack, including nematodes (Fosu-Nyarko et al. 2009), virus (Hamada et al. 2008) and bacteria (Kottapalli et al. 2007). It seems that this protein has a common role in the response to diverse pathogens and could be triggering the HR. In the present study, the upregulation of AsPHI1 at 9 DAI in nematode-inoculated roots of A. stenosperma coincided with the previously demonstrated appearance of HR (Proite et al. 2008). Another defence-related protein, lipocalin (AsLIP) showed gradual decline of expression during nematode infection. This suggests that this lipocalin homolog might act as a scavenger for the products of the oxidative stress during HR (Charron et al. 2008).
Likewise, plant genes containing the U-BOX (PUB) domain have been demonstrated to be upregulated under pathogen infection (Yee and Goring 2009) or after elicitor treatment, such as chitin, found in nematode exoskeleton (Libault et al. 2007). The silencing of ubiquitin-related genes reduced the HR in tobacco and pepper, whereas its over-expression enhanced disease resistance in Arabidopsis (González-Lamothe et al. 2006; Lee et al. 2011). Here, we identified a PUB (AsU-BOX) gene in roots of A. stenosperma, which was the gene most upregulated during the early stages of infection. This gene showed a gradual and significant increase in its relative expression along the bioassay time course, from 4-fold at 3 DAI to 17-fold at 9 DAI (Fig. 1). These results are in accordance with our previous macroarray analysis (Guimarães et al. 2010), reinforcing the important role that this protein may exert in mediating the HR.
Another protein family containing a specific domain related to plant defence is the DUF protein family, identified in Arabidopsis, rice and tomato plants grown under various environmental stress conditions, such as nutrient deficiency, Agrobacterium-induced crown gall, and mixed elicitors (Brunings et al. 2009; Gholizadeh 2011). DUF protein was shown to elevate activity of enzymes that induce the HR response, such as catalase, peroxidase, polyphenol oxidase and phenyalanine ammonia lyase, when applied to tobacco leaves (Gholizadeh 2011). In this study, AsDUF was upregulated in the three time points studied, suggesting its role in the induction of these ROS enzymes. Catalases play an important role in the detoxification of ROS in the cell, and its inhibition allows hydrogen peroxide level to be increased, thus, triggering HR (Molinari 1998). The upregulation of AsCAT that we observed in the three time points analysed was compatible with the occurrence of the HR and consequently cell death around 9 DAI, as previously described in A. stenosperma (Proite et al. 2008).
Membrane/cell wall components
Upon root-knot nematode infection, host cells are transformed into multinucleated feeding sites – the giant cells. The molecular mechanisms underlying this reprogramming of root cell fate to acquire functions that benefit nematodes include cell wall modification and extensions by proteins such as expansin, integrins, tetraspanin and xyloglucan endotransglycosylase (Davis and Mitchum 2005; Wieczorek and Seifert 2012).
The expression profiles of two genes encoding interacting plasma membrane proteins, an integrin and a tetraspanin, involved in cell adhesion and signal transduction (Bassani and Cingolani 2012), were also investigated in this study. The role of these two proteins in cell signalling and plasma membrane–cell wall junction integrity in the root-knot nematode giant cell formation involves a restructuring of plasma membrane and cell wall continuum. We showed that both genes exhibited the same expression profile, being downregulated at 3 DAI, but with no significant differential expression afterwards. These results are in agreement with Tirumalaraju et al. (2011), who showed that there was no increase expression of these genes in the peanut nematode resistant cultivar Nematam, but an upregulation in the susceptible cultivar Florunner. Similarly, we observed a xyloglucan endotransglycosylase homolog (AsXET) was downregulated at all the three time points. Therefore, we hypothesise that the low expression of these three genes in the early stages of nematode infection in the resistant A. stenosperma could interfere with the cell wall loosening and elongation process necessary for gall formation.
Hormonal balance
Endoparasitic nematodes induce hormonal changes in the host, contributing to the formation of giant cells. Auxin signalling is essential for the formation and maintenance of the root-gall (reviewed by Goverse et al. 2000; Bird and Kaloshian 2003). However, less is known about genes that are downregulated by auxin, such as auxin repressed protein gene (ARP), with some of them related to biotic and abiotic stresses and plant development (Song et al. 2007; Salvianti et al. 2008).
Previous analyses by macroarray and northern blotting showed that AsARP was differentially expressed between infected and control roots of A. stenosperma and also in susceptible A. hypogaea roots (Guimarães et al. 2010). Our present data corroborate the expression profile of AsARP in A. stenosperma. In qRT-PCR analysis, AsARP showed initial downregulation at 3 DAI followed by a gradual increase in expression as nematode infection progressed. Considering that the AsARP expression is inversely related to the accumulation of auxin, our data are compatible with a similar study in soybean, suggesting that after an initial auxin increase in the developing giant-cells, root-knot nematodes decrease auxin biosynthesis in feeding cells at later stages of the infection, thus, altering plant cell development (Doyle and Lambert 2003). AsARP transcripts were detected by in situ hybridisation in cortical and xylem cells and, after gene upregulation, an increase in the concentration of transcripts was observed in these same cells. This confirms the specific localisation of these transcripts in cells that are largely involved in water transportation into the central cylinder of the root, mostly through diffusion.
In parallel to auxins, cytokinins also have been shown to play an important role in root-knot nematode gall formation, with high levels of expression induced in the early stages of plant-parasite interaction (Goverse et al. 2000; Barcala et al. 2010). Cytokinin dehydrogenase (CKX) expression is induced by cytokinins, representing a negative feedback system, in which the accumulation of cytokinin induces its own catabolism, controlling the levels of the hormone in the cell (Lee et al. 2007). Lohar et al. (2004) showed that CKX overexpression in Lotus hairy roots reduced gall formation, indicating that the initial stages of gall cell must require high levels of cytokinins.
In our study, AsCKX clearly showed, by both qRT-PCR and in situ hybridisation, a diminishing expression during the first days of nematode infection, especially at 3 and 6 DAI. This corroborated our previous macroarray and northern blotting data that showed AsCKX downregulation, especially in contrast with the susceptible A. hypogaea (Guimarães et al. 2010). We suggest that, as in A. stenosperma, there was no induction of AsCKX because the feeding site was not established due to HR, and, as previously shown, there was no increase in the influx of cytokinin in the cells surrounding the nematode feeding site (Lohar et al. 2004).
Conclusively, in situ data correlated with those obtained by qRT-PCR for the two hormonal balance related genes (AsARP and AsCKX) studied during the early phases of the infection. Their involvement in the differentiation of plant cells into nematode feeding sites will be studied further in plant models to evaluate their potential as candidate genes for peanut transformation and nematode resistance improvement.
Conclusion
The modulation of most gene expressions analysed here is in accordance with the occurrence of the HR, which is the resistance mechanism employed by A. stenosperma. The fact that genes with differential expression profiles representing different steps of the defence response-recognition pathogen signal, intracellular signalling, transcriptional activation, cell wall and membrane modification, hormone rebalance and production of secondary metabolites, reassures the functionality of the resistance network.
To validate these gene functions and understand their role in the HR response of A. stenosperma to M. arenaria race 1 interaction, they will be over-expressed/silenced in model plants (Medicago trunculata and Arabidopsis) or in composite peanut plants with transgenic Agrobacterium rhizogenes roots. The built up knowledge in these candidate genes is of great interest for engineering nematode resistance in peanut.
Acknowledgements
The authors gratefully acknowledge CNPq, FAP-DF, The Challenge Program Generation, Tropical Legume Improvement (TL1) and host institutions for funding this research. The authors also wish to thanks Teresa Mullens (University of California Riverside) for assistance in the bioassay and Sheng Zhao (Stanford University, California) for the help with PCR Miner tool.
References
Agrios GN (2005) ‘Plant pathology.’ (Elsevier Academic Press: Amsterdam)Albuquerque E, Carneiro R, Costa P, Gomes A, Santos M, Pereira A, Nicole M, Fernandez D, Grossi-de-Sa M (2010) Resistance to Meloidogyne incognita expresses a hypersensitive-like response in Coffea arabica. European Journal of Plant Pathology 127, 365–373.
| Resistance to Meloidogyne incognita expresses a hypersensitive-like response in Coffea arabica.Crossref | GoogleScholarGoogle Scholar |
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. Journal of Molecular Biology 215, 403–410.
Anthony F, Topart P, Martinez A, Silva M, Nicole M (2005) Hypersensitive-like reaction conferred by the Mex-1 resistance gene against Meloidogyne exigua in coffee. Plant Pathology 54, 476–482.
| Hypersensitive-like reaction conferred by the Mex-1 resistance gene against Meloidogyne exigua in coffee.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVSltr3F&md5=d04f24749eab3ec2b86be5dce5a155e8CAS |
Barcala M, García A, Cabrera J, Casson S, Lindsey K, Favery B, García-Casado G, Solano R, Fenoll C, Escobar C (2010) Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. The Plant Journal 61, 698–712.
| Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivVShu7g%3D&md5=9739e019d0de660d90920133c6f8a734CAS | 20003167PubMed |
Bassani S, Cingolani LA (2012) Tetraspanins: interactions and interplay with integrins. International Journal of Biochemistry & Cell Biology 44, 703–708.
| Tetraspanins: interactions and interplay with integrins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XisFKisrs%3D&md5=84d969c5bbf0180b2a136659380c13cbCAS |
Bendezu IF, Starr JL (2003) Mechanism of resistance to Meloidogyne arenaria in the peanut cultivar COAN. Journal of Nematology 35, 115–118.
Bird DM, Kaloshian I (2003) Are roots special? Nematodes have their say. Physiological and Molecular Plant Pathology 62, 115–123.
| Are roots special? Nematodes have their say.Crossref | GoogleScholarGoogle Scholar |
Brunings AM, Datnoff LE, Ma JF, Mitani N, Nagamura Y, Rathinasabapathi B, Kirst M (2009) Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Annals of Applied Biology 155, 161–170.
| Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ylsb7J&md5=18cb49ef7e3b70047172debce828b46eCAS |
Cacas J-L, Marmey P, Montillet J-L, Sayegh-Alhamdia M, Jalloul A, Rojas-Mendoza A, Clérivet A, Nicole M (2009) A novel patatin-like protein from cotton plant, GhPat1, is co-expressed with GhLox1 during Xanthomonas campestris-mediated hypersensitive cell death. Plant Cell Reports 28, 155–164.
| A novel patatin-like protein from cotton plant, GhPat1, is co-expressed with GhLox1 during Xanthomonas campestris-mediated hypersensitive cell death.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVylsr%2FJ&md5=b0a9333d566aa985f8d03730853b7b10CAS | 18850102PubMed |
Castillo MB, Morrison LS, Russell CC, Banks DJ (1973) Resistance to Meloidogyne hapla in peanut. Journal of Nematology 5, 281–285.
Chang X, Heene E, Qiao F, Nick P (2011) The phytoalexin resveratrol regulates the initiation of hypersensitive cell death in Vitis cell. PLoS ONE 6, e26405
| The phytoalexin resveratrol regulates the initiation of hypersensitive cell death in Vitis cell.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVyqu7%2FJ&md5=5d5062b732a72ac78b7fafdf1a611703CAS | 22053190PubMed |
Charron J-B, Ouellet F, Houde M, Sarhan F (2008) The plant apolipoprotein D ortholog protects Arabidopsis against oxidative stress. BMC Plant Biology 8, 86
| The plant apolipoprotein D ortholog protects Arabidopsis against oxidative stress.Crossref | GoogleScholarGoogle Scholar | 18671872PubMed |
Choi K, Burow MD, Church G, Burow G, Paterson AH, Simpson CE, Starr JL (1999) Genetics and mechanism of resistance to Meloidogyne arenaria in peanut germplasm. Journal of Nematology 31, 283–290.
Chung I-M, Park MR, Chun JC, Yun SJ (2003) Resveratrol accumulation and resveratrol synthase gene expression in response to abiotic stresses and hormones in peanut plants. Plant Science 164, 103–109.
| Resveratrol accumulation and resveratrol synthase gene expression in response to abiotic stresses and hormones in peanut plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XovFertrs%3D&md5=d913b5628d987bb88baf38dae664e1ffCAS |
Collange BA, Navarrete M, Peyre GL, Mateille T, Tchamitchian M (2011) Root-knot nematode (Meloidogyne) management in vegetable crop production: the challenge of an agronomic system analysis. Crop Protection 30, 1251–1262.
| Root-knot nematode (Meloidogyne) management in vegetable crop production: the challenge of an agronomic system analysis.Crossref | GoogleScholarGoogle Scholar |
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411, 826–833.
| Plant pathogens and integrated defence responses to infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXksF2gu74%3D&md5=512c428d4fdc146b8711ca4221c85c73CAS | 11459065PubMed |
Das S, Ehlers J, Close T, Roberts P (2010) Transcriptional profiling of root-knot nematode induced feeding sites in cowpea (Vigna unguiculata L. Walp.) using a soybean genome array. BMC Genomics 11, 480
| Transcriptional profiling of root-knot nematode induced feeding sites in cowpea (Vigna unguiculata L. Walp.) using a soybean genome array.Crossref | GoogleScholarGoogle Scholar | 20723233PubMed |
Davis EL, Mitchum MG (2005) Nematodes. sophisticated parasites of legumes. Plant Physiology 137, 1182–1188.
| Nematodes. sophisticated parasites of legumes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjslaqtbs%3D&md5=da67ad0c2349d69f0bed7f055b2419dbCAS | 15824280PubMed |
Davis EL, Meyers DM, Burton JW, Barker KR (1998) Resistance to root-knot, reniform, and soybean cyst nematodes in selected soybean breeding lines. Journal of Nematology 30, 530–541.
de Sá MEL, Lopes MJC, de Araújo Campos M, Paiva LV, dos Santos RMA, Beneventi MA, Firmino AAP, de Sá MFG (2012) Transcriptome analysis of resistant soybean roots infected by Meloidogyne javanica. Genetics and Molecular Biology 35, 272–282.
| Transcriptome analysis of resistant soybean roots infected by Meloidogyne javanica.Crossref | GoogleScholarGoogle Scholar |
Dhondt S, Geoffroy P, Stelmach BA, Legrand M, Heitz T (2000) Soluble phospholipase A2 activity is induced before oxylipin accumulation in tobacco mosaic virus-infected tobacco leaves and is contributed by patatin-like enzymes. The Plant Journal 23, 431–440.
| Soluble phospholipase A2 activity is induced before oxylipin accumulation in tobacco mosaic virus-infected tobacco leaves and is contributed by patatin-like enzymes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXms1eht7s%3D&md5=e0f8618504a92ae0b4718fef610ee912CAS | 10972869PubMed |
Doyle EA, Lambert KN (2003) Meloidogyne javanica chorismate mutase 1 alters plant cell development. Molecular Plant-Microbe Interactions 16, 123–131.
| Meloidogyne javanica chorismate mutase 1 alters plant cell development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmtFelsQ%3D%3D&md5=c1572359fbcf26206427ebea8edac6d9CAS | 12575746PubMed |
Fernandez-Calvo P, Chini A, Fernández-Barbero G, Chico J-M, Gimenez-Ibanez S, Geerinck J, Eeckhout D, Schweizer F, Godoy M, Franco-Zorrilla JM, Pauwels L, Witters E, Puga MI, Paz-Ares J, Goossens A, Reymond P, De Jaeger G, Solano R (2011) The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. The Plant Cell 23, 701–715.
| The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvVOrs74%3D&md5=527ef3084a766ef15a6348455f10c940CAS | 21335373PubMed |
Flor HH (1946) Genetics of pathogenicity in Melampsora lini. Journal of Agricultural Research 73, 335–357.
Fosu-Nyarko J, Jones MGK, Wang Z (2009) Functional characterization of transcripts expressed in early-stage Meloidogyne javanica-induced giant cells isolated by laser microdissection. Molecular Plant Pathology 10, 237–248.
| Functional characterization of transcripts expressed in early-stage Meloidogyne javanica-induced giant cells isolated by laser microdissection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjslalt78%3D&md5=96bbc7127a3ecc24ccdbcb8aa5c517acCAS | 19236572PubMed |
Gholizadeh A (2011) Heterologous expression of stress-responsive DUF538 domain containing protein and its morpho-biochemical consequences. The Protein Journal 30, 351–358.
| Heterologous expression of stress-responsive DUF538 domain containing protein and its morpho-biochemical consequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotVCmtrY%3D&md5=4a1f27842c92be6f48df93177a883aedCAS | 21710148PubMed |
González-Lamothe R, Tsitsigiannis DI, Ludwig AA, Panicot M, Shirasu K, Jones JDG (2006) The U-Box protein CMPG1 is required for efficient activation of defense mechanisms triggered by multiple resistance genes in tobacco and tomato. The Plant Cell 18, 1067–1083.
| The U-Box protein CMPG1 is required for efficient activation of defense mechanisms triggered by multiple resistance genes in tobacco and tomato.Crossref | GoogleScholarGoogle Scholar | 16531490PubMed |
Goverse A, de Almeida Engler J, Verhees J, van der Krol S, Helder J, Gheysen G (2000) Cell cycle activation by plant parasitic nematodes. Plant Molecular Biology 43, 747–761.
| Cell cycle activation by plant parasitic nematodes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXosVGksbw%3D&md5=407bf3cfc8cc61e12bbaa4efa287bf6bCAS | 11089874PubMed |
Graham MA, Marek LF, Lohnes D, Cregan P, Shoemaker RC (2000) Expression and genome organization of resistance gene analogs in soybean. Genome 43, 86–93.
| Expression and genome organization of resistance gene analogs in soybean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhs1yhs70%3D&md5=1aa8163463421bb8a8be7d779b754023CAS | 10701117PubMed |
Guimarães PM, Brasileiro ACM, Proite K, de Araújo ACG, Leal-Bertioli SCM, Pic-Taylor A, da Silva FR, Morgante CV, da Graça Ribeiro S, Bertioli DJ (2010) A study of gene expression in the nematode resistant wild peanut relative, Arachis stenosperma, in response to challenge with Meloidogyne arenaria. Tropical Plant Biology 3, 183–192.
| A study of gene expression in the nematode resistant wild peanut relative, Arachis stenosperma, in response to challenge with Meloidogyne arenaria.Crossref | GoogleScholarGoogle Scholar |
Hamada H, Matsumura H, Tomita R, Terauchi R, Suzuki K, Kobayashi K (2008) SuperSAGE revealed different classes of early resistance response genes in Capsicum chinense plants harboring L3-resistance gene infected with Pepper mild mottle virus. Journal of General Plant Pathology 74, 313–321.
| SuperSAGE revealed different classes of early resistance response genes in Capsicum chinense plants harboring L3-resistance gene infected with Pepper mild mottle virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFOmt7jJ&md5=c43cbfb3b579845ee437cc9e7fe23d55CAS |
Heim MA, Jakoby M, Werber M, Martin C, Weisshaar B, Bailey PC (2003) The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Molecular Biology and Evolution 20, 735–747.
| The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvFCgu78%3D&md5=acdc4730b74b0488c539f103d1d9b26aCAS | 12679534PubMed |
Holbrook CC, Stephenson MG, Johnson AW (2000) Level and geographical distribution of resistance to Meloidogyne arenaria in the US peanut germplasm collection. Crop Science 40, 1168–1171.
| Level and geographical distribution of resistance to Meloidogyne arenaria in the US peanut germplasm collection.Crossref | GoogleScholarGoogle Scholar |
Huang CS (1985) Formation, anatomy and physiology of giant cells induced by root-knot nematodes. In ‘An advanced treatise on meloidogyne’. (Eds JN Sasser, CC Carter) pp. 155–164. (North Carolina State University Graphics: Raleigh, NC, USA)
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter 57, 1025–1028.
Ibrahim H, Hosseini P, Alkharouf N, Hussein E, Gamal El-Din AEK, Aly M, Matthews B (2011) Analysis of gene expression in soybean (Glycine max) roots in response to the root knot nematode Meloidogyne incognita using microarrays and KEGG pathways. BMC Genomics 12, 220
| Analysis of gene expression in soybean (Glycine max) roots in response to the root knot nematode Meloidogyne incognita using microarrays and KEGG pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtlKlsL4%3D&md5=7c104c1ff75cafa27f6388a8cdd32291CAS | 21569240PubMed |
Jammes F, Lecomte P, de Almeida-Engler J, Bitton F, Martin-Magniette M-L, Renou JP, Abad P, Favery B (2005) Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. The Plant Journal 44, 447–458.
| Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1alsbnL&md5=cc590e29801050ee28df54ecb607d856CAS | 16236154PubMed |
Jones MGK (1981) Host cell responses to endoparasitic nematode attack: structure and function of giant cells and syncytia. Annals of Applied Biology 97, 353–372.
| Host cell responses to endoparasitic nematode attack: structure and function of giant cells and syncytia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXitVGgtb8%3D&md5=280b5a798a14b8453589f25b6e96c40aCAS |
Kottapalli KR, Rakwal R, Satoh K, Shibato J, Kottapalli P, Iwahashi H, Kikuchi S (2007) Transcriptional profiling of indica rice cultivar IET8585 (Ajaya) infected with bacterial leaf blight pathogen Xanthomonas oryzae pv oryzae. Plant Physiology and Biochemistry 45, 834–850.
| Transcriptional profiling of indica rice cultivar IET8585 (Ajaya) infected with bacterial leaf blight pathogen Xanthomonas oryzae pv oryzae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFGrsL7E&md5=d8fbc979bf7204772c94c5f987d513faCAS | 17870590PubMed |
Kyndt T, Denil S, Haegeman A, Trooskens G, Bauters L, Van Criekinge W, De Meyer T, Gheysen G (2012) Transcriptional reprogramming by root knot and migratory nematode infection in rice. New Phytologist 196, 887–900.
| Transcriptional reprogramming by root knot and migratory nematode infection in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVKrt7vJ&md5=77a5d48b5d6a9439b30a317fb9228949CAS | 22985291PubMed |
Lam E (2004) Controlled cell death, plant survival and development. Nature Reviews. Molecular Cell Biology 5, 305–315.
| Controlled cell death, plant survival and development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXivV2itro%3D&md5=788b5620ca1ab6bb483c573177e005cdCAS | 15071555PubMed |
Lee DJ, Park JY, Ku SJ, Ha YM, Kim S, Kim MD, Oh MH, Kim J (2007) Genome-wide expression profiling of ARABIDOPSIS RESPONSE REGULATOR 7(ARR7) overexpression in cytokinin response. Molecular Genetics and Genomics 277, 115–137.
| Genome-wide expression profiling of ARABIDOPSIS RESPONSE REGULATOR 7(ARR7) overexpression in cytokinin response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1GrtLY%3D&md5=cb1df4f2a2f85a5b7c4ece577315fbe6CAS | 17061125PubMed |
Lee DH, Choi HW, Hwang BK (2011) The pepper E3 ubiquitin ligase RING1 gene, CaRING1, is required for cell death and the salicylic acid-dependent defense response. Plant Physiology 156, 2011–2025.
| The pepper E3 ubiquitin ligase RING1 gene, CaRING1, is required for cell death and the salicylic acid-dependent defense response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVOrur3N&md5=f816532bfe43da7467cb029077da4047CAS | 21628629PubMed |
Libault M, Wan J, Czechowski T, Udvardi M, Stacey G (2007) Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Molecular Plant-Microbe Interactions 20, 900–911.
| Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1ems74%3D&md5=565ef0a8fdf15ba3eb222d1033c3a5c5CAS | 17722694PubMed |
Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM (2004) Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbioses. The Plant Journal 38, 203–214.
| Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbioses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXkt1Kksrw%3D&md5=450ac1c90820dec3b3ed598100792a9bCAS | 15078325PubMed |
Marum L, Miguel A, Ricardo CP, Miguel C (2012) Reference gene selection for quantitative real-time PCR normalization in Quercus suber. PLoS ONE 7, e35113
| Reference gene selection for quantitative real-time PCR normalization in Quercus suber.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmsVGgtLs%3D&md5=b15962467d9f4f7d8303f4bb5c1ffc86CAS | 22529976PubMed |
Meyers BC, Kaushik S, Nandety RS (2005) Evolving disease resistance genes. Current Opinion in Plant Biology 8, 129–134.
| Evolving disease resistance genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXitVCntLk%3D&md5=86a40296b6af3cfd443b7fb63d1fb200CAS | 15752991PubMed |
Molinari S (1998) Changes of catalase and SOD activities in the early response of tomato to Meloidogyne attack. Nematologia Mediterranea 26, 167–172.
Morel J-B, Dangl JL (1997) The hypersensitive response and the induction of cell death in plants. Cell Death and Differentiation 4, 671–683.
| The hypersensitive response and the induction of cell death in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnvF2qs7c%3D&md5=3ca4b429d203cc7edf739c1bf2f5e188CAS | 16465279PubMed |
Morgante CV, Guimaraes PM, Martins ACQ, Araujo ACG, Leal-Bertioli SC, Bertioli D, Brasileiro ACM (2011) Reference genes for quantitative reverse transcription-polymerase chain reaction expression studies in wild and cultivated peanut. BMC Research Notes 4, 339
| Reference genes for quantitative reverse transcription-polymerase chain reaction expression studies in wild and cultivated peanut.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1GjurjI&md5=2565d13fa18166776e2eaa638b85b0dcCAS | 21906295PubMed |
Mota FC, Alves GCS, Giband M, Gomes ACMM, Sousa FR, Mattos VS, Barbosa VHS, Barroso PAV, Nicole M, Peixoto JR, Rocha MR, Carneiro RMDG (2012) New sources of resistance to Meloidogyne incognita race 3 in wild cotton accessions and histological characterization of the defence mechanisms. Plant Pathology
| New sources of resistance to Meloidogyne incognita race 3 in wild cotton accessions and histological characterization of the defence mechanisms.Crossref | GoogleScholarGoogle Scholar |
Nagy E, Chu Y, Guo Y, Khanal S, Tang S, Li Y, Dong WB, Timper P, Taylor C, Ozias-Akins P, Holbrook CC, Beilinson V, Nielsen NC, Stalker HT, Knapp SJ (2010) Recombination is suppressed in an alien introgression in peanut harboring Rma, a dominant root-knot nematode resistance gene. Molecular Breeding 26, 357–370.
| Recombination is suppressed in an alien introgression in peanut harboring Rma, a dominant root-knot nematode resistance gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpt1aluro%3D&md5=9d814a7b936ce78851f2d7d1007d5ddfCAS |
Nelson SC, Starr JL, Simpson CE (1990) Expression of resistance to Meloidogyne arenaria in Arachis batizocoi and A. cardenasii. Journal of Nematology 22, 242–244.
Niu Y, Figueroa P, Browse J (2011) Characterization of JAZ-interacting bHLH transcription factors that regulate jasmonate responses in Arabidopsis. Journal of Experimental Botany 62, 2143–2154.
| Characterization of JAZ-interacting bHLH transcription factors that regulate jasmonate responses in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFyisr8%3D&md5=79acc626feff016bcc53ac7a94c6cd0aCAS | 21321051PubMed |
Olowe T (2009) Cowpea germplasm resistant to Meloidogyne arenaria race 1, Meloidogyne incognita race 4 and Meloidogyne javanica. European Journal of Scientific Research 28, 338–350.
Orlowska E, Basile A, Kandzia I, Llorente B, Kirk HG, Cvitanich C (2012) Revealing the importance of meristems and roots for the development of hypersensitive responses and full foliar resistance to Phytophthora infestans in the resistant potato cultivar Sarpo Mira. Journal of Experimental Botany 63, 4765–4779.
| Revealing the importance of meristems and roots for the development of hypersensitive responses and full foliar resistance to Phytophthora infestans in the resistant potato cultivar Sarpo Mira.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1KjsLfJ&md5=14df4a85b71fe11d14487effb1fa5bcdCAS | 22844094PubMed |
Pegard A, Brizzard G, Fazari A, Soucaze O, Abad P, Djian-Caporalino C (2005) Histological characterization of resistance to different root-knot nematode species related to phenolics accumulation in Capsicum annuum. Phytopathology 95, 158–165.
| Histological characterization of resistance to different root-knot nematode species related to phenolics accumulation in Capsicum annuum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsFaisb4%3D&md5=8a38a5b40dea7bcbd0677991b95f121dCAS | 18943985PubMed |
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research 30, e36
| Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR.Crossref | GoogleScholarGoogle Scholar | 11972351PubMed |
Portillo M, Cabrera J, Lindsey K, Topping J, Andrés MF, Emiliozzi M, Oliveros JC, García-Casado G, Solano R, Koltai H, Resnick N, Fenoll C, Escobar C (2013) Distinct and conserved transcriptomic changes during nematode-induced giant cell development in tomato compared with Arabidopsis: a functional role for gene repression. New Phytologist 197, 1276–1290.
| Distinct and conserved transcriptomic changes during nematode-induced giant cell development in tomato compared with Arabidopsis: a functional role for gene repression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVOjuro%3D&md5=5c74ab3e8f9652562d6c0696bd6f8a6dCAS | 23373862PubMed |
Proite K, Leal-Bertioli S, Bertioli D, Moretzsohn M, da Silva F, Martins N, Guimarães P (2007) ESTs from a wild Arachis species for gene discovery and marker development. BMC Plant Biology 7, 7
| ESTs from a wild Arachis species for gene discovery and marker development.Crossref | GoogleScholarGoogle Scholar | 17302987PubMed |
Proite K, Carneiro R, Falcão R, Gomes A, Leal-Bertioli S, Guimarães P, Bertioli D (2008) Post-infection development and histopathology of Meloidogyne arenaria race 1 on Arachis spp. Plant Pathology 57, 974–980.
| Post-infection development and histopathology of Meloidogyne arenaria race 1 on Arachis spp.Crossref | GoogleScholarGoogle Scholar |
Salvianti F, Bettini PP, Giordani E, Sacchetti P, Bellini E, Buiatti M (2008) Identification by suppression subtractive hybridization of genes expressed in pear (Pyrus spp.) upon infestation with Cacopsylla pyri (Homoptera: Psyllidae). Journal of Plant Physiology 165, 1808–1816.
| Identification by suppression subtractive hybridization of genes expressed in pear (Pyrus spp.) upon infestation with Cacopsylla pyri (Homoptera: Psyllidae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVKgurbK&md5=fc030d6aa8c81655d00948efb208b6ecCAS | 18343531PubMed |
Shinya T, Gális I, Narisawa T, Sasaki M, Fukuda H, Matsuoka H, Saito M, Matsuoka K (2007) Comprehensive analysis of glucan elicitor-regulated gene expression in tobacco BY-2 cells reveals a novel MYB transcription factor involved in the regulation of phenylpropanoid metabolism. Plant & Cell Physiology 48, 1404–1413.
| Comprehensive analysis of glucan elicitor-regulated gene expression in tobacco BY-2 cells reveals a novel MYB transcription factor involved in the regulation of phenylpropanoid metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlems7nE&md5=cb79d4f0041c40d922c980b21158c340CAS |
Sobolev VS (2008) Localized production of phytoalexins by peanut (Arachis hypogaea) kernels in response to invasion by Aspergillus species. Journal of Agricultural and Food Chemistry 56, 1949–1954.
| Localized production of phytoalexins by peanut (Arachis hypogaea) kernels in response to invasion by Aspergillus species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisVWltbY%3D&md5=8628a4353e0afa6766e8812b1cee9505CAS | 18298071PubMed |
Song YJ, Joo JH, Ryu HY, Lee JS, Bae YS, Nam KH (2007) Reactive oxygen species mediate IAA-induced ethylene production in mungbean (Vigna radiata L) hypocotyls. Journal of Plant Biology 50, 18–23.
| Reactive oxygen species mediate IAA-induced ethylene production in mungbean (Vigna radiata L) hypocotyls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXktleitLw%3D&md5=6e64ef6a2f187bc43991537b6d28fac2CAS |
Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M (2010) A practical approach to RT-qPCR – publishing data that conform to the MIQE guidelines. Methods 50, S1–S5.
| A practical approach to RT-qPCR – publishing data that conform to the MIQE guidelines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtVCnsrs%3D&md5=cbcd7759f902c111f877e1bc0aa5628dCAS | 20215014PubMed |
Tirumalaraju SV, Jain M, Gallo M (2011) Differential gene expression in roots of nematode-resistant and -susceptible peanut (Arachis hypogaea) cultivars in response to early stages of peanut root-knot nematode (Meloidogyne arenaria) parasitization. Journal of Plant Physiology 168, 481–492.
| Differential gene expression in roots of nematode-resistant and -susceptible peanut (Arachis hypogaea) cultivars in response to early stages of peanut root-knot nematode (Meloidogyne arenaria) parasitization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlaqtLo%3D&md5=167e424b072369b0a18dce2badfe51f3CAS | 20863592PubMed |
Tornero P, Merritt P, Sadanandom A, Shirasu K, Innes RW, Dangl JL (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. The Plant Cell 14, 1005–1015.
| RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XksVGqsbY%3D&md5=7942811a478fa076b2518c4d182c0a9fCAS | 12034893PubMed |
Tucker MR, Araujo A-CG, Paech NA, Hecht V, Schmidt EDL, Rossell J-B, de Vries SC, Koltunow AMG (2003) Sexual and apomictic reproduction in Hieracium subgenus pilosella are closely interrelated developmental pathways. The Plant Cell 15, 1524–1537.
| Sexual and apomictic reproduction in Hieracium subgenus pilosella are closely interrelated developmental pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlslWktLc%3D&md5=987ac321bb2c8bb1a0606170d7f3c80cCAS | 12837944PubMed |
Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Research 35, W71–W74.
| Primer3Plus, an enhanced web interface to Primer3.Crossref | GoogleScholarGoogle Scholar | 17485472PubMed |
Vos P, Simons G, Jesse T, Wijbrandi J, Heinen L, Hogers R, Frijters S, Groenendijk J, Diergaarde P, Reijans M, Fierens-Onstenk J, de Both M, Peleman J, Liharska T, Hontelez J, Zabeau M (1998) The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nature Biotechnology 16, 1365–1369.
| The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnslOlsrY%3D&md5=2ca59888466f90f14a1bcb080176c39fCAS | 9853621PubMed |
Wang X (2004) Lipid signaling. Current Opinion in Plant Biology 7, 329–336.
| Lipid signaling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVamsLk%3D&md5=f739f3ca27bd3023fa3db34a4d5f2598CAS | 15134755PubMed |
Wieczorek K, Seifert GJ (2012) Plant cell wall signaling in the interaction with plant-parasitic nematodes. In ‘Biocommunication of plants’. (Eds W Günther, B František) pp. 139–155. (Springer: Heidelberg, Germany)
Williamson VM, Gleason CA (2003) Plant–nematode interactions. Current Opinion in Plant Biology 6, 327–333.
| Plant–nematode interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlsValtL8%3D&md5=dd7adeb2d6a35a533f64206978d968c7CAS | 12873526PubMed |
Williamson VM, Kumar A (2006) Nematode resistance in plants: the battle underground. Trends in Genetics 22, 396–403.
| Nematode resistance in plants: the battle underground.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmslyksLo%3D&md5=1467fd3ad094d44e7a320fe83f025207CAS | 16723170PubMed |
Yang W, Devaiah SP, Pan X, Isaac G, Welti R, Wang X (2007) AtPLAI is an acyl hydrolase involved in basal jasmonic acid production and Arabidopsis resistance to Botrytis cinerea. Journal of Biological Chemistry 282, 18 116–18 128.
| AtPLAI is an acyl hydrolase involved in basal jasmonic acid production and Arabidopsis resistance to Botrytis cinerea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsVOlurw%3D&md5=a5e724f5520481cac41dda271ae4eec0CAS |
Yang M-H, Kuo C-H, Hsieh W-C, Ku K-L (2010) Investigation of microbial elicitation of trans-resveratrol and trans-piceatannol in peanut callus led to the application of chitin as a potential elicitor. Journal of Agricultural and Food Chemistry 58, 9537–9541.
| Investigation of microbial elicitation of trans-resveratrol and trans-piceatannol in peanut callus led to the application of chitin as a potential elicitor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVSisr7N&md5=1d82ad04f2e7090417f03c12f7f580b6CAS | 20704182PubMed |
Yee D, Goring DR (2009) The diversity of plant U-box E3 ubiquitin ligases: from upstream activators to downstream target substrates. Journal of Experimental Botany 60, 1109–1121.
| The diversity of plant U-box E3 ubiquitin ligases: from upstream activators to downstream target substrates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjsFShsbY%3D&md5=2e6b1aa54655298af7abdb706fafd867CAS | 19196749PubMed |
Zhao S, Fernald RD (2005) Comprehensive algorithm for quantitative real-time polymerase chain reaction. Journal of Computational Biology 12, 1047–1064.
| Comprehensive algorithm for quantitative real-time polymerase chain reaction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFemtb7K&md5=159cd28b2b30d6ae5d6cbc7abde070a1CAS | 16241897PubMed |


