Awn primordium to tipping is the most decisive developmental phase for spikelet survival in barley
Ahmad M. Alqudah A and Thorsten Schnurbusch A BA Research Group Plant Architecture, Genebank Department, Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben 06466, Germany.
B Corresponding author. Email: thor@ipk-gatersleben.de
Functional Plant Biology 41(4) 424-436 https://doi.org/10.1071/FP13248
Submitted: 22 August 2013 Accepted: 7 October 2013 Published: 18 November 2013
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
In small-grain cereals, grain yield is closely associated with grain number. Improved spikelet survival is an important trait for increasing grain yield. We investigated spikelet number, spikelet survival and yield-related traits under greenhouse conditions, and pot- and soil-grown field conditions. Thirty-two spring barley (Hordeum vulgare L.) accessions (14 two- and 18 six-rowed accessions) were manually dissected to determine spikelet/floret number on the main culm spike (SNS) at awn primordium (AP), tipping (TIP), heading and anther extrusion. We observed a significant difference between two- and six-rowed barley for SNS and spikelet survival at all stages and growing conditions. Both traits were highly genetically controlled, with repeatability and broad-sense heritability values of 0.74–0.93. The rate of spikelet survival from AP to harvest was higher in two- (~70%) than in six-rowed (~58%) barley. Spikelet abortion, starting immediately after AP, was negatively affected by increased SNS and the thermal time required to reach the AP stage. The largest proportion of spikelet reduction happened during the AP–TIP phase, which was the most critical period for spikelet survival. The duration between AP and the end of stem elongation correlated better with spikelet survival and yield-related characters than the estimated duration of stem elongation using leaf height measurements. Our observations indicate that the main spike plays an important role in single-plant grain yield. Extending the length of the critical AP–TIP phase is promising for improving yield through increased spikelet development and survival. The results also demonstrate that greenhouse conditions are appropriate for studying traits such as phase duration and spikelet survival in barley.
Additional keywords: Hordeum vulgare L., six-rowed, spikelet survival, stem elongation, two-rowed.
Introduction
Barley (Hordeum vulgare L.) is considered to be the fourth most important cereal food crop in the world (Food and Agriculture Organisation 2012), largely due to its exceptional adaptations towards growing in a variety of different environmental conditions. The barley spike possesses three single-flowered spikelets (one central and two lateral spikelets) at each rachis internode (Forster et al. 2007). Based on the fertility of the lateral spikelets, barley has been classified into two different row types, namely the two- and six-rowed barleys. All three spikelets are fertile in six-rowed barley, but the two lateral spikelets are sterile in two-rowed barley (Bonnett 1966). The difference in lateral spikelet fertility between the two germplasm pools is one of the major factors determining barley yield potential.
Improved grain yield is a major objective of crop breeding, and a promising avenue for maximising yield is through improved spikelet survival. Several researchers have considered the pre-anthesis development phase as a target for improving yield potential (Appleyard et al. 1982; Kitchen and Rasmusson 1983; Borràs et al. 2009). However, little is known about spikelet survival and its role in improving grain yield. Crop breeding programs have focussed intensively on final grain yield directly rather than improving other yield components such as spikelet survival. In barley, the number of spikelets per spike at the awn primordium (AP) stage represents the maximum yield potential per spike (Riggs and Kirby 1978; Waddington et al. 1983; Kirby and Appleyard 1987; Kernich et al. 1997). Moreover, the maximum number of spikelet or floret primordia in wheat (Triticum aestivum L.) is genetically controlled (Kirby et al. 1989; González et al. 2003). Six-rowed barley has more fertile spikelet/floret primordia per spike at the AP stage than two-rowed barley does (Whingwiri and Stern 1982; Kirby and Appleyard 1987; Kernich et al. 1997; Miralles et al. 2000; del Moral et al. 2002; Arisnabarreta and Miralles 2006) and variation in the number of spikelets per spike at AP is higher in six-rowed barley (Kitchen and Rasmusson 1983; Kernich et al. 1997).
Several studies have postulated that the differences in spikelet mortality between two- and six-rowed barley arise as a result of competition for assimilates (Kirby 1988; Arisnabarreta and Miralles 2004), competition between spikelets per spike (Appleyard et al. 1982) or the position of spikelets within the spike (Arisnabarreta and Miralles 2006). Spikelet abortion is generally higher in six-rowed barleys (Frank et al. 1992; Kernich et al. 1997; Arisnabarreta and Miralles 2004; Arisnabarreta and Miralles 2006) because they possess more fertile spikelet primordia per spike (Whingwiri and Stern 1982; Kirby and Appleyard 1987; Kernich et al. 1997; Miralles et al. 2000; del Moral et al. 2002; Arisnabarreta and Miralles 2006). However, no focussed research has been performed to identify the causes of spikelet and floret survival from AP to harvest in both row type classes of barley.
In barley, the pre-anthesis developmental phases include the vegetative phase (leaf initiation), the early reproductive phase (spikelet or floret initiation; from the double ridge to AP) and the late reproductive phase (spike growth and development; from AP to anthesis) (Appleyard et al. 1982; Kirby and Appleyard 1987; Slafer and Rawson 1994; Sreenivasulu and Schnurbusch 2012). Variation in the duration of the pre-anthesis developmental phases and morphological changes, particularly during the late reproductive phase, have been reported (Appleyard et al. 1982; Kitchen and Rasmusson 1983; Kernich et al. 1995b; Kernich et al. 1997). The duration of these phases is affected by environmental conditions such as temperature, photoperiod and vernalisation, but also by genotypic differences among barley varieties (Appleyard et al. 1982; Kernich et al. 1995a; Kernich et al. 1997). Increasing the duration between the triple mound stage (when the spikelet ridge part of the double ridge has differentiated into three distinct bumps or mounds (Kirby and Appleyard 1987)) and heading time (HD) may increase barley grain yield through higher spikelet fertility (Miralles et al. 2000). In barley and wheat, stem elongation (SE) appeared to be the critical period for determining spikelet survival (Cottrell et al. 1985; González et al. 2003) and extending its duration has been proposed as an appropriate way for increasing grain yield (Miralles et al. 2000). Therefore a more complete understanding of the genetic constitution of these pre-anthesis phases could help breeders in improving grain yield (del Moral et al. 2002).
In this study, we investigated spikelet survival from the standpoint of developing a more detailed description of the later reproductive phases in barley and their specific influences on fertility. We sought to identify a subphase in which the majority of spikelets were aborted and to estimate the broad-sense heritability of this trait. Finally, we examined differences in spikelet survival between two- and six-rowed barleys as well as in plants growing in different environments (greenhouse vs. field). In our study of spikelet fertility in the context of later reproductive development, we used 32 diverse spring barley accessions to address the following specific objectives: (i) to quantify spikelet number per main culm spike (SNS) at several stages during the late reproductive phase (from AP to anther extrusion (AE)) and to determine spikelet survival in individual subphases; (ii) to measure yield, yield components and the contribution of the main spike to single-plant yield; (iii) to estimate the genetic basis for these traits by calculating their repeatability or broad-sense heritability; and (iv) to correlate the onset of SE and the late reproductive subphases with spikelet survival in barley.
Analyses of these traits in 32 accessions under greenhouse and field conditions showed that spikelet survival in barley is highly genetically controlled. We narrowed down the critical growing period in which most spikelet abortion occurred and found that most of the reductions consistently happened during the first subphase of the late reproductive phase, from AP to tipping (TIP), regardless of different growing conditions. These findings may help breeders to maximise barley grain yield by focussing on this critical period of grain development.
Materials and methods
Plant materials and experimental conditions
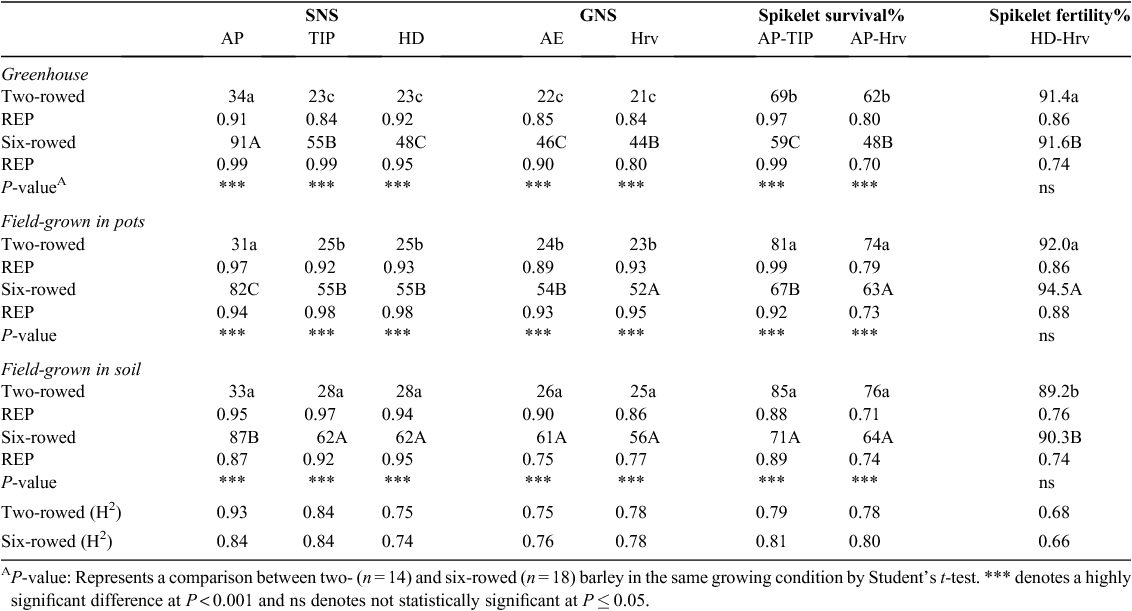
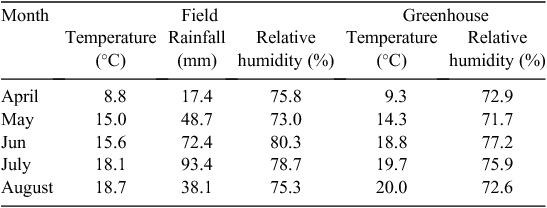
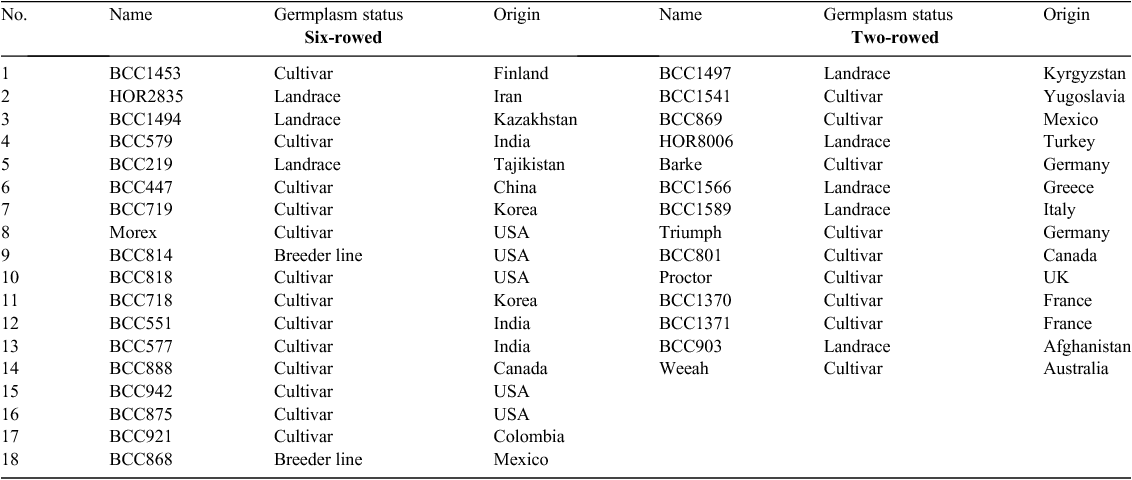
The study was conducted at the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben, Germany. Three growing conditions were used during this study: (i) greenhouse, with planting into pots in controlled conditions; (ii) field planting into pots and (iii) field planting into soil. Temperature, rainfall and humidity data for the field and greenhouse conditions are presented in Table 1. Seeds for each of the three growing conditions were planted on the same date (1st of April 2012) and each condition was represented as a set of 32 spring barley (Hordeum vulgare L.) accessions comprising a worldwide collection of 14 two-rowed and 18 six-rowed accessions (see Table 2). Thirty plants per accession were planted in each growing condition, resulting in a total of 960 barley plants. Agricultural practices were performed as recommended, including pest, disease and weed control.
|
|
For the greehouse experiment (long days, 16 h : 8 h day : night and ~20°C : ~16°C day : night), seeds were germinated in trays and seedlings were grown for 10 days until they reached the two- to three-leaf stage. Seedlings were then exposed to vernalisation at ~4°C for a period of 28 days. Seedlings were subsequently hardened for a period of 7 days to gradually acclimatise the plants under 12 h : 12 h and ~14°C : ~12°C for day : night and temperature, respectively. Plants were transplanted into 0.5-L pots (one plant per pot; 9 × 9 cm pot diameter and height) and grown in potting substrate (peatmoss fertilised with 14 : 16 : 18 N : P : K) under long-day conditions (16 h : 8 h light : dark and ~20°C : ~16°C). Plants were irrigated daily and each 0.5-L pot was fertilised with 1.5 g (17 : 11 : 10 N : P : K) fertiliser to avoid nutritional deficiencies. Supplemental light (~300 mEm–2 s–1 PAR) was used to extend the natural light with low-intensity incandescent lamps (Philips son-t agro 400 w). Randomisation of pots was conducted three times per week to minimise border and temperature gradient effects on growth and development.
For pot-grown plants in the field, seeds were directly planted into identical 0.5-L pots. Plants were grown in potting substrate and fertilised as above. Plants were manually irrigated when required.
For soil-grown plants in the field, seeds were planted directly into silty loam soil (10 plants per row; rows were 50 cm long with 20-cm spacing between rows). Three rows for each accession were randomly distributed and 15 g of fertiliser per row (17 : 11 : 10 N : P : K) was applied. Plants were manually irrigated when required.
Phenotyping and data recording
Data were collected at five developmental stages: AP (Z 31-33, this stage is reached when the tip of the lemma starts to grow and curves over the stamen primordia (maximum yield potential) (Kirby and Appleyard 1987)), TIP (Z49, first awns visible from the flag leaf sheath), heading (HD,Z55, the inflorescence has half emerged from the flag leaf sheath), AE (Z65, the spikes have anthers extruded) and physiological maturity (Z92, yellow spike) (Zadoks et al. 1974). The time for each stage was recorded when at least 50% of the main culm spike in each accession had reached this stage. Growing degree days (GDD) or thermal time was used to identify the required temperature for each stage and the base temperature was set to 0°C.
Initially, three plants per accession were randomly selected and tagged when the youngest leaf (i.e. the top leaf) on the main culm had initiated (Kiss et al. 2011). Estimations of the onset, length and end of SE were calculated by measuring the height of the youngest fully developed leaf (the distance between the soil surface and the youngest leaf) eight times. These measurements started from Z20 (main shoot only) and continued to Z69 (anthesis completed) (Zadoks et al. 1974) and are expressed as thermal time as well as the number of days. SE started to occur when the distance between the soil surface and the youngest leaf increased; SE reached an end when this distance stopped changing. To identify the critical duration for spikelet survival, SE was calculated in two different ways: (1) the estimated onset of SE (based on leaf height) to the end of SE, and (2) from AP to the end of SE. Correlations with grain yield, yield components and spikelet survival using both methods were calculated.
During the first four developmental stages (AP, TIP, HD and AE), three plants per accession were randomly selected to determine each stage and the number of spikelet primordia or spikelets on each main culm. To determine the AP stage accurately, immature barley inflorescences were prepared for microscopic dissection and image capture (Stereo Microscope Stemi 2000-C with KL 1500 LCD; Axio Vision, 4.8.2, ZEISS Germany) to count the number of spikelet primordia from each main culm. To identify the exact timing for the AP stage (Kirby and Appleyard 1987), regular dissection of the main culm apex had to be performed in each accession three times per week. Floral primordia were counted along the spike to score the maximum SNS.
The number of spikelets was counted at each stage to deduce the changes in SNS. For each sample, main spike dry weight (MSDW) and tiller numbers were also recorded. At each stage, SNS and its relation to GDD was calculated to identify the importance of GDD in spikelet survival between different stages. Spikelet survival was calculated based on the total number of spikelets (both sterile and fertile) and spikelet fertility was calculated based on the yield of fertile grain at harvest. The equations for spikelet survival and fertility are shown in Eqns 1 and 2:
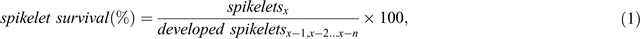
where x is a specific stage, and x – 1, x – 2… x – n are stages prior to x. For example, if x is harvest then x – 1 is AP and x – 2 is TIP.
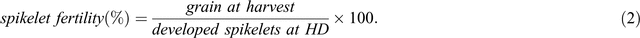
Yield and yield components
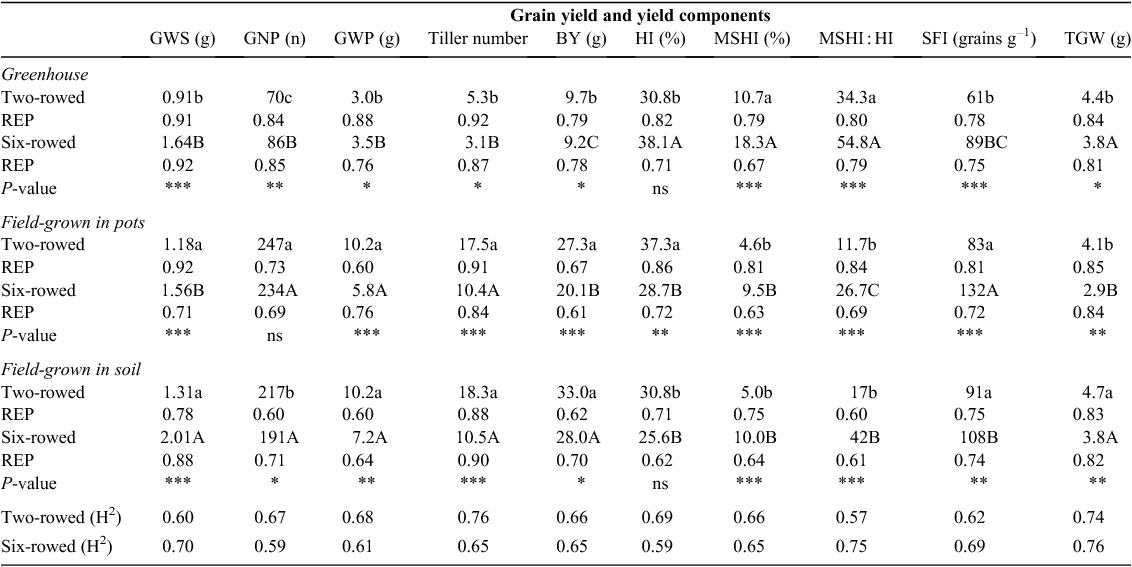
Six plants per accession were randomly harvested by hand to determine biological yield (BY), which was expressed as the total weight of air-dried aboveground tissue. Single-plant grain yield and yield components were measured by counting the number of grains per main spike (SNS) and per plant (GNP), and total grain weight per main spike (GWS) and per plant (GWP) following hand threshing. Harvest index (HI) per plant was measured as the ratio of grain weight per plant to BY per plant multiplied by a factor of 100. Main spike harvest index (MSHI) was measured as the ratio of GWS to BY per plant. The ratio of MSHI to total HI of each plant was measured to identify the contribution of the main spike towards grain yield. The spike fertility index (SFI) was calculated as the ratio between the number of grains per gram of the main culm spike (GNS) to the dry weight of the main culm spike chaff (non-grain biomass of the spike) at harvest (grains g–1). The grain weight of 1000 grains (1000-grain weight or TGW) was measured at harvest.
Statistical analyses
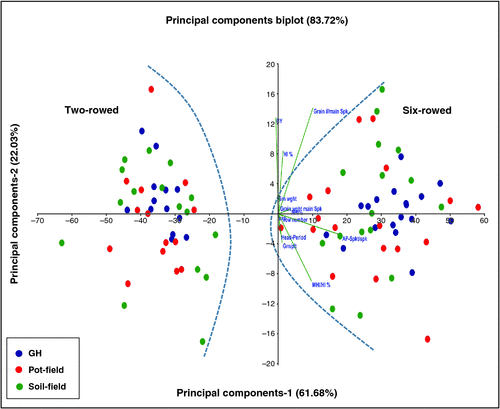
Each growing condition had a completely randomised design with three replications for each stage and six replications for single-plant yield and yield components. The collected data were analysed using SAS for Windows ver. 9.3 SAS Institute Inc., Cary, NC, USA at the probability level P ≤ 0.05. Student’s t-test was used to compare between row types (i.e. two- and six-rowed) from the same growing condition, and Fisher’s LSD was used to compare row types across growing conditions. Phenotypic correlation analyses (Pearson) among row types and growing conditions were calculated using PROC CORR (SAS 2012). The repeatability of individual traits was calculated for each row type, with growing condition and broad-sense heritability across growing conditions as the ratio between genetic and the phenotypic variance components (PROC VARCOMP; SAS Institute Inc.). Multivariate analysis was performed by principal component analysis (PCA; biplot) to interpret and summarise the major pattern of variation among growing conditions by accessions by heading date, yield and yield components (main culm spike and single plant). PCA is an indicator ordination tool for obtaining multivariate data that can be explored visually in a two-dimensional PCA correlation biplot. PCA was calculated based on accession means for each trait under each growing condition to study the inter-relationships among the components using Genstat for Windows ver. 16 (VSN International, Hemel Hempstead, UK).
Results
Comparisons among growing conditions
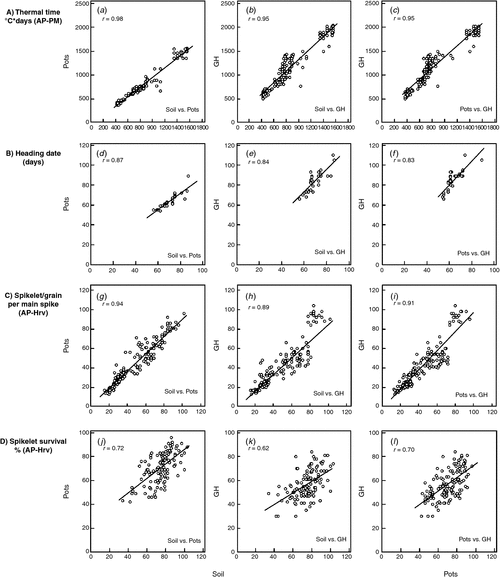
To verify whether greenhouse conditions can be used to study spikelet survival and related traits of interest, we performed a correlation analysis of growing conditions for spike-related traits with data obtained from both greenhouse-grown and field-grown plants. Correlations among growing conditions for thermal time, heading date, spikelet number and spikelet survival per main spike showed significant to extremely robust correlations ranging from 0.62 to 0.98 (Fig. 1). The highest correlation among different growing conditions was observed for thermal time from sowing to physiological maturity (r ≥ 0.95), whereby the strongest correlation was observed between soil-grown and pot-grown field plants (r = 0.98). Correlations among growing conditions were r ≥ 0.89 for SNS (from AP to harvest) and r ≥ 0.83 for heading date. Moreover, soil-grown and pot-grown field plants had the strongest correlations among the growing conditions (r = 0.87 and r = 0.94 for SNS and heading date, respectively). However, spikelet survival had the lowest correlation among different growing conditions and ranged from 0.62 (soil vs. greenhouse) to 0.72 (soil vs. pot). For the four traits analysed, a general trend was apparent, showing slightly higher correlations between soil-grown and pot-grown field plants and slightly lower correlations between soil-grown field plants and greenhouse-grown plants. To validate results from the correlation analyses, we also performed multivariate analysis of the 32 barley accessions using PCA (Fig. 2). The PCA analysis was used to examine whether growing conditions had any effects on plant development, yield and yield components. Based upon PCA 1 and PCA 2, two groups could be clearly identified due to their differences in row type (two- and six-rowed barleys). In each row type group, accessions from different growing conditions clearly overlapped with no further clustering, suggesting that row type differences explained most of the observed variation in these experiments.
|
|
Thermal time to reach developmental stages, subphases and SE
Comparisons of thermal time to reach different developmental stages in two- and six-rowed barley under the same growing condition yielded no significant differences between the row type classes (P ≤ 0.05; Fig. 3a). The duration from sowing to reach each developmental stage was significantly longer in both row type classes under greenhouse conditions compared with pot-grown and soil-grown field plants (Fig. 3a). Regardless of row type, the pot-grown field plants showed the most rapid development in all stages before AE. In two-rowed barley, the duration of developmental stages was significantly different among growing conditions at AP, TIP and HD (Fig. 3a). Very similar trends were found for the six-rowed barley results at all stages at P ≤ 0.05 (Fig. 3a). The duration between AP and TIP was the longest reproductive subphase in comparison to GDD between TIP and HD, and between HD and AE under all growing conditions and row types (Fig. 3b). The shortest reproductive subphases for both row type classes was in pot-grown field plants in the AP–TIP and TIP–HD stages. However, soil-grown field plants had the shortest duration between HD and AE (Fig. 3b). Generally, there was no significant difference between two- and six-rowed barley in terms of the duration of the subphases in plants growing under the same conditions.
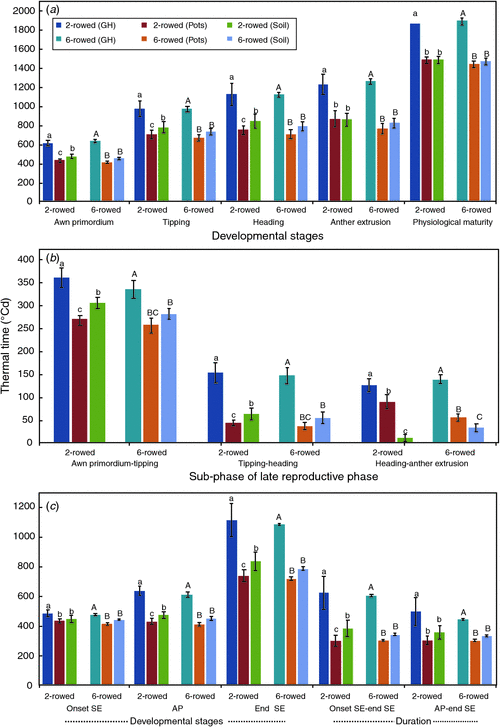
|
Differences between both row types for the onset of SE, AP, the end of SE and the duration of SE in the same growing conditions were not significant (Fig. 3c). Spikes from greenhouse-grown plants required more thermal time to reach each stage than plants under pot- and soil-grown field conditions. There were no significant differences between the onset of SE and AP in both row type classes under field conditions but differences of 124 GDD and 161 GDD in two- and six-rowed barleys were observed under greenhouse conditions (Fig. 3c). SE halted approximately at the HD stage under all growing conditions. The duration from the onset to the end of SE and from AP to the end of SE was significantly longer under greenhouse conditions compared with pot- and soil-grown field conditions.
The correlations of the duration between the estimated onset of SE (based on leaf height measurements) to the end of SE and AP to the end of SE (GDD) with spikelet survival and yield components traits were calculated for all growing conditions. The correlations for GNS at harvest, spikelet survival from AP to harvest, MSDW at HD, GWS, GNP and GWP ranged from 0.11 to 0.82 for the estimated onset of SE to the end of SE (Table 3). A very similar range of correlations was obtained for the duration from AP to the end of SE (0.16 to 0.88) for both row types under all growing conditions (Table 3). However, the correlation was higher for spikelet survival in AP to the end of SE (r = 0.68 for two- and six-rowed barley under greenhouse and field-grown pot conditions) than the duration from the estimated onset (leaf height measurement) to the end of SE (r = 0.58 for six-rowed barley under field-grown pot conditions). For MSDW at HD, the highest correlation was for the two-rowed barley under greenhouse conditions (r = 0.75 for AP to end of SE and r = 0.73 for the onset to the end of SE). The highest correlation value for GWS was obtained from two-rowed barley under pot-grown conditions (r = 0.87 for AP to the end of SE compared with r = 0.82 for the estimated onset to the end of SE). For GNP, the correlation between the duration of AP to the end of SE was higher than the correlation between the duration of the estimated onset to the end of SE (r = 0.71 and r = 0.65, respectively). For GWP, the highest correlation (r = 0.68) was observed for the duration of AP to the end of SE compared with the onset to the end of SE (r = 0.57). There was a trend of higher correlations with spikelet survival and yield traits with the duration starting from AP compared with the estimated onset of SE (based upon leaf height).
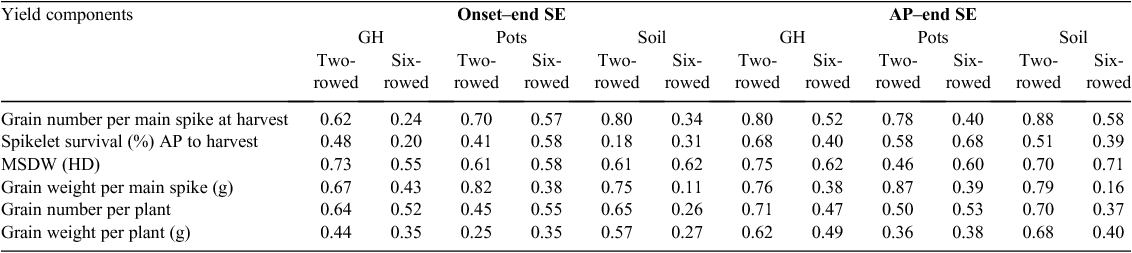
|
SNS, spikelet survival and fertility
Under all growing conditions, there were highly significant differences (P ≤ 0.001) between both row type classes for SNS and spikelet survival. Across the three growing conditions, six-rowed barley generally had significantly higher SNS at all stages compared with two-rowed barley (Table 4). In all growing conditions, two-rowed barley had similar numbers of spikelet primordia at the AP stage, but showed a strong decline in SNS from AP to TIP. After this developmental period, only a gradual reduction of SNS was observed. Significant differences for the highest SNS among growing conditions were found after AP in soil-grown field plants, followed by pot-grown field plants and greenhouse-grown plants (Table 4). In six-rowed barley, greenhouse-grown plants had the highest yield potential measured as SNS at AP. The reduction in SNS between AP and TIP was evident under greenhouse conditions, but at TIP, the spikes from greenhouse-grown plants and pot-grown field plants displayed the same number of spikelets (Table 4). Consequently, spikelet reduction mostly occurred from AP to TIP in both row type classes under all growing conditions, with the highest relative reduction occurring in greenhouse-grown plants (Table 4). Two-rowed barley had significantly higher spikelet survival than six-rowed barley at all stages and in all growing conditions (Table 4). Spikelet survival from AP to TIP in two-rowed barley was significantly higher in soil-grown and pot-grown field plants than in GH-grown plants. A higher spikelet survival rate was observed in six-rowed barley from AP-TIP in soil-grown field plants, followed by pot-grown field plants and greenhouse-grown plants (Table 4). In comparisons across all stages, spikelet survival in two- and six-rowed barley in soil-grown and pot-grown field plants was significantly higher than in greenhouse-grown plants (Table 4). There was no significant difference between two- and six-rowed barley in spikelet fertility in plants growing under the same conditions. Spikelet fertility from HD to harvest was significantly lower in soil-grown field plants than in pot-grown field and greenhouse-grown plants (Table 4). Notably, the repeatability and broad-sense heritability values for SNS and spikelet survival ranged from 0.70 to 0.99, indicating that these traits are highly heritable under all growing conditions (Table 4).
Grain yield and major yield components per plant
Analysis of yield and yield components obtained from main culm spikes and single plants showed that there are significant differences among row type classes and growing conditions. GWS and grain weight per plant (GWP) were significantly different for row-types in the different growing conditions (Table 5). Six-rowed spikes had significantly higher GWS than two-rowed spikes under all growing conditions. In contrast, two-rowed barley had significantly higher GWP in pot- and soil-grown field plants. GWS and GWP in greenhouse-grown plants were significantly lower than that in pot- and soil-grown field plants. In general, GNS and GNP were significantly lower under greenhouse conditions (Tables 4 and 5).There were significant differences in tiller number per plant among row types and growing conditions (Table 5). Two-rowed barley produced significantly more tillers per plant (13.7 ± 5.2) than six-rowed barley (8.0 ± 3.5). Moreover, greenhouse-grown plants had significantly fewer tillers per plant (4.2 ± 1.5) compared with pot- (14.0 ± 4.1) and soil-grown field plants (14.4 ± 3.9). There were significant differences among row type and growing conditions for BY (Table 5). Average BY was higher in two-rowed barley under all growing conditions compared with six-rowed barley. Pot- and soil-grown field plants generated a higher BY regardless of row type. The lower BY in greenhouse-grown plants was mostly attributed to fewer tillers, reduced grain number and reduced grain weight (Table 5). There were significant differences between row types and among growing conditions for HI. Generally, HI was higher in greenhouse-grown plants than in pot- and soil-grown field plants, and two-rowed barley had a higher HI than six-rowed barley (Table 5). By using GNS, GWS and BY data, we found that the MSHI and the ratio of MSHI to HI were higher in six-rowed barley under all growing conditions, suggesting that the six-rowed spike contributed more to single-plant yield than MSHI for two-rowed barley. Furthermore, both traits were higher in greenhouse-grown plants than in pot- and soil-grown plants of both row types. Notably, GWS was the most important contributor to increased HI, particularly under greenhouse conditions. As a consequence of higher GNS in six-rowed barley, SFI at harvest was significantly higher in six-rowed barley under all growing conditions. Pot- and soil-grown plants generally showed a higher SFI than greenhouse-grown plants (Table 5). TGW was also significantly different between row types (Table 5). Two-rowed barley produced significantly higher TGW than six-rowed barley under all growing conditions. The analysis of TGW among growing conditions showed that in two-rowed barley, soil-grown plants had significantly higher TGW compared with the other growing conditions. In contrast, pot-grown plants showed significantly lower TGW in six-rowed barley (Table 5). Repeatability values for grain yield and yield components were above 0.6 for each growing condition, but broad-sense heritability values were smaller across growing conditions.
Discussion
This study supports the assertion that the maximum yield potential in barley occurs at the AP stage (Kirby and Appleyard 1987). Spikelet number declined after AP, resulting in significant differences for spikelet survival and final GNS between the two row type classes (Fig. 4). Moreover, in the present study, we identified AP to TIP as being the most critical subphase related to spikelet reduction and grain yield per main spike (Fig. 4). The main culm spike also had a significant role in improving yield potential. In addition, the duration between AP and the end of SE showed better correlations with yield and yield components than did the estimated onset of SE (leaf height measurement).
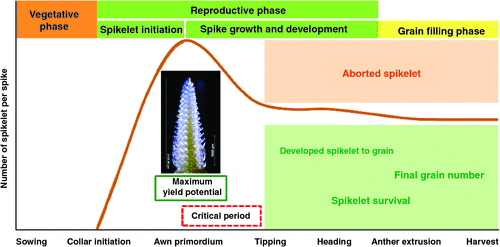
|
Maximum yield potential and spikelet survival in the two row type classes
In this study, we examined the degree of variation in spikelet survival during spike development in different barley row types and determined its repeatability and broad-sense heritability under different growing conditions. In both row type classes, the mortality of the spikelet primordia started with the onset of fast stem and spike growth under all conditions and lasted until HD, and this finding is consistent with the findings of other groups (Kirby 1988; Miralles et al. 2000; Arisnabarreta and Miralles 2006). Regardless of the specific growing condition, we found that from all initiated spikelets, ~70% and 58% of spikelets survived in two-rowed and six-rowed barley, respectively. Therefore, six-rowed barley had higher spikelet mortality than two-rowed barley. Arisnabarreta and Miralles (2006) reported slightly lower spikelet survival in near isogenic lines of two- and six-rowed barley (63% and 44%) in comparison to the present study. Several groups have suggested reasons for the differences in spikelet survival observed between two- and six-rowed barley. For example, six-rowed barley possesses a greater number of potentially fertile spikelet primordia compared with two-rowed barley at the AP stage. Because of the greater sink size and competition among spikelets within a spike, the majority of spikelet primordia are aborted in six-rowed barley (Appleyard et al. 1982). Moreover, Arisnabarreta and Miralles (2006) explained the differences between barley row types with respect to spikelet survival based on spikelet structure and position. These authors noted that the smaller carpels in six-rowed barley may be a cause for reduced spikelet survival. Moreover, they found that the reduced synchrony among central, basal and apical spikelet primordia explained higher spikelet survival in two-rowed barley. In wheat, most of the floret abortion was observed in more distal spikelets (Whingwiri and Stern 1982), possibly due to separated vascular bundles between the rachis and distal spikelets (Hanif and Langer 1972). We propose that similar mechanisms for higher spikelet survival in two-rowed barley are at play in our study.
We also showed that the abortion of spikelets was more pronounced under greenhouse conditions than in pot- and soil-grown field plants. This observation could be explained by the increased interval of time required to reach AP under greenhouse conditions. This possibly resulted in a longer spike differentiation phase and hence, in the production of more spikelet primordia. An increase in the number of spikelet primordia or SNS under greenhouse conditions may have led to increased within-spike competition, which, in turn, resulted in a higher proportion of aborted spikelets.
We demonstrated that spikelet survival was similar under all growing conditions (environments) and that it is highly genetically controlled. The high broad-sense heritability suggests a promising unexplored opportunity to better understand the genetic basis of spikelet survival in barley, thereby opening up a new area of research for increasing yield potential.
Effect of subphases on spike growth, development and spikelet survival
The importance of strictly defining pre-anthesis phases for improving the yield potential of barley has been previously suggested (Ellis and Kirby 1980; Kitchen and Rasmusson 1983; del Moral et al. 2002). However, few studies have explored the specific contribution of pre-anthesis subphases to spikelet survival. In this study, we tested whether dividing spike growth and development into subphases can reveal a critical subphase that is important for spikelet mortality. Miralles et al. (2000) reported that the period between the triple mound and HD is important for yield in barley and extending this period might effectively increase spikelet fertility. Our analysis showed that the AP–TIP subphase is the most critical period for spikelet survival, where more than two-thirds of the reduction in spikelet number occurred, regardless of the environment in which the plants were grown. This finding further narrows down the critical spikelet survival phase to a very precise interval contained within the longer phase reported by Miralles et al. (2000). Our results, therefore, support the notion that the period before HD is the most crucial for spikelet abortion (Kernich et al. 1996). The causes for spikelet abortion are unclear, but it is likely that the duration from AP toTIP is not sufficient to allow most of the newly initiated spikelet primordia to become fertile. The early phases of SE also coincide with spikelet development, which possibly drains resources away from developing and growing spikes. The duration between AP and HD in barley is sensitive to photoperiod. In some cases, main culm spikelet primordia were aborted when plants were grown under long days; this is likely to be due to the shortening of the spikelet development period under long photoperiods (Kernich et al. 1996). Our study was also conducted under long-day conditions, suggesting that photoperiod contributed to the higher spikelet abortion observed between the AP and TIP stages of development.
Importance of the main culm spike in improving yield
The main culm spike in barley, which is formed earlier than the secondary spikes, was the greatest contributor to single-plant grain yield and is therefore a worthwhile target to improve spikelet survival. The importance of the main culm spike in single-plant grain yield lies in producing more and heavier grains compared with spikes from side tillers in spring barley (Cottrell et al. 1985). Due to wheat breeding programs over the last 20 years, SNS and grain number per spikelet have been improved by more than 30% through specific genetic gains in grain number on the main spike (Sanchez-Garcia et al. 2013). In our study, we tested the contribution of the main culm spike using distinct parameters and found that yield from the main culm spike, measured as MSHI or the ratio of MSHI to HI, is clearly higher in six-rowed barley than in two-rowed barley. This observation is very probably due to the higher GNS, higher GWS and lower number of spikes and tillers per plant in six-rowed barley, resulting in a relatively higher MSHI. From an agronomical point of view, both GNS and GWS were highly correlated with GNP and GWP in all growing conditions. Thus, improving single-plant grain yield through targeted improvements of the main culm spike will be an important future goal.
Start and duration of SE, and its correlation with yield and yield components
One objective of the present study was to correlate yield components with (i) the duration from AP to the end of SE (the late reproductive phase) and (ii) the duration of the estimated onset to the end of SE (based on leaf height Kiss et al. (2011). A previously reported method to estimate the onset of SE made use of the appearance of the first node on the stem (Borràs et al. 2009). However, Kiss et al. (2011) noted that this method leads to an inflated interval between the first node’s appearance and the onset of SE in spring barley. In our study, we calculated that the estimated onset of SE occurred ~3–4 days earlier than the average occurrence of AP across all row types and different growing conditions. Positive correlations were found between the duration of SE and all traits, including spikelet survival (Table 3). The importance of SE for spikelet survival and grain yield has been studied in small-grain cereals (Fischer 2007; Miralles and Slafer 2007). Kernich et al. (1996) reported that spikelet survival is negatively affected under long-day conditions due to a shortened SE period, and this was also a likely consideration in our study. As shown in Table 3, the duration between AP to the end of SE had better correlations than the length of time between the estimated onset to the end of SE with spikelet survival and yield traits. Manipulating vegetative and reproductive phases independently, particularly by extending the reproductive phase, is likely to increase the number of fertile florets by increasing assimilate acquisition by the spikes in barley and wheat (Slafer and Rawson 1994; Kernich et al. 1996; Miralles et al. 2000). It seems that this particular interval is most critical because the survival of the initiated spikelets is determined during this period, which, in turn, represents the final grain number. Despite the importance of the duration of this phase (i.e. AP to TIP), it has received little attention in the literature, probably because this type of study requires high-quality microscopic dissection of spikes.
Summary and conclusion
Our study of barley accessions grown in different environments revealed that, firstly, maximum yield potential in both row types was observed at the AP stage of development, and the difference in spikelet survival between two-rowed and six-rowed barely is significant. Secondly, spikelet abortion occurred at a rate of ~30% in two-rowed barley and 42% in six-rowed barley. The majority of spikelet abortion occurred during the AP–TIP phase, which coincides with SE. More than two-thirds (~72%) of all aborted spikelets were aborted during this phase, emphasising the importance of this phase for improving barley yield. Thirdly, the duration of the interval from AP to the end of SE is a better indicator than the estimated onset to the end of SE duration for spikelet survival and grain yield in barley. Fourth, the main culm spike is a major contributor to single-plant yield, particularly in six-rowed barley. Fifth, all of the growing conditions used in this study were suitable for studying agronomical and developmental traits such as flowering time, phase durations and spikelet survival. Lastly, the results from this study also indicated that spikelet survival in barley is highly genetically controlled and an in-depth analysis of this trait is a worthwhile target for increasing yield in barley.
Acknowledgements
The authors acknowledge Dr Benjamin Kilian and the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) Genebank for providing seeds from diverse barley accessions. We also thank Prof Nezar Samarah and Ravi Koppolu for critically reading previous versions of the manuscript. Special thanks go to Mrs. Annett Beyer for her excellent technical assistance. We also thank Dr Ammar Albalasmeh and Mrs Karin Lipfert for graphical design and the IPK staff supervised by Mrs. Kathrin Gramel-Eikenroth for their help during this work. This study was financially supported by Deutsche Forschungsgemeinschaft grant number SCHN 768/4–1 and German Federal Ministry of Education and Research GABI-FUTURE Start Program grant number 0315071 to TS.
References
Appleyard M, Kirby JM, Fellowes G (1982) Relationship between duration of phases in the pre-anthesis life cycle of spring barley. Australian Journal of Agricultural Research 33, 917–925.| Relationship between duration of phases in the pre-anthesis life cycle of spring barley.Crossref | GoogleScholarGoogle Scholar |
Arisnabarreta S, Miralles DJ (2004) The influence of fertiliser nitrogen application on development and number of reproductive primordia in field-grown two- and six-rowed barleys. Australian Journal of Agricultural Research 55, 357–366.
| The influence of fertiliser nitrogen application on development and number of reproductive primordia in field-grown two- and six-rowed barleys.Crossref | GoogleScholarGoogle Scholar |
Arisnabarreta S, Miralles DJ (2006) Floret development and grain setting in near isogenic two- and six-rowed barley lines (Hordeum vulgare L.). Field Crops Research 96, 466–476.
| Floret development and grain setting in near isogenic two- and six-rowed barley lines (Hordeum vulgare L.).Crossref | GoogleScholarGoogle Scholar |
Bonnett OT (1966) Inflorescences of maize, wheat, rye, barley, and oats: their initiation and development. University of Illinois College of Agriculture, Agricultural Experiment Station Bulletin 721, 59–77.
Borràs G, Romagosa I, van Eeuwijk F, Slafer GA (2009) Genetic variability in duration of pre-heading phases and relationships with leaf appearance and tillering dynamics in a barley population. Field Crops Research 113, 95–104.
| Genetic variability in duration of pre-heading phases and relationships with leaf appearance and tillering dynamics in a barley population.Crossref | GoogleScholarGoogle Scholar |
Cottrell JE, Easton RH, Dale JE, Wadsworth AC, Adam JS, Child RD, Hoad GV (1985) A comparison of spike and spikelet survival in mainstem and tillers of barley. Annals of Applied Biology 106, 365–377.
| A comparison of spike and spikelet survival in mainstem and tillers of barley.Crossref | GoogleScholarGoogle Scholar |
del Moral LG, Miralles DJ, Slafer G (2002) Initiation and appearance of vegetative and reproductive structures throughout barley development. In ‘Barley science: recent advances from molecular biology to agronomy of yield and quality’. (Eds GA Slafer, JL Molina-Cano, R Savin, JL Araus and I Romagosa) pp. 243–267 (Food Product Press: New York)
Ellis RP, Kirby EJM (1980) A comparison of spring barley grown in England and in Scotland. 2. Yield and its components. The Journal of Agricultural Science 95, 111–115.
| A comparison of spring barley grown in England and in Scotland. 2. Yield and its components.Crossref | GoogleScholarGoogle Scholar |
Fischer RA (2007) Understanding the physiological basis of yield potential in wheat. The Journal of Agricultural Science 145, 99–113.
| Understanding the physiological basis of yield potential in wheat.Crossref | GoogleScholarGoogle Scholar |
Forster BP, Franckowiak JD, Lundqvist U, Lyon J, Pitkethly I, Thomas WT (2007) The barley phytomer. Annals of Botany 100, 725–733.
| The barley phytomer.Crossref | GoogleScholarGoogle Scholar | 17901062PubMed |
Frank AB, Bauer A, Black AL (1992) Effects of air-temperature and fertilizer nitrogen on spike development in spring barley. Crop Science 32, 793–797.
| Effects of air-temperature and fertilizer nitrogen on spike development in spring barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XltFSktbc%3D&md5=aabea8fc4de8eb0c18e0c6d630d071d9CAS |
Food and Agriculture Organisation (FAO) (2012) Information on post-harvesting operations. Available online at: http://www.fao.org/inpho/inpho-post-harvest-compendium/cereals-grains/en/ [Verified 30 October 2013].
González FG, Slafer GA, Miralles DJ (2003) Floret development and spike growth as affected by photoperiod during stem elongation in wheat. Field Crops Research 81, 29–38.
| Floret development and spike growth as affected by photoperiod during stem elongation in wheat.Crossref | GoogleScholarGoogle Scholar |
Hanif M, Langer RHM (1972) Vascular system of spikelet in wheat (Triticum aestivum). Annals of Botany 36, 721–727.
Kernich GC, Halloran GM, Flood RG (1995a) Variation in developmental patterns of wild barley H. spontaneum and cultivated barley. Euphytica 82, 105–115.
| Variation in developmental patterns of wild barley H. spontaneum and cultivated barley.Crossref | GoogleScholarGoogle Scholar |
Kernich GC, Slafer GA, Halloran GM (1995b) Barley development as affected by rate of change of photoperiod. The Journal of Agricultural Science 124, 379–388.
| Barley development as affected by rate of change of photoperiod.Crossref | GoogleScholarGoogle Scholar |
Kernich G, Halloran G, Flood R (1996) Constant and interchanged photoperiod effects on the rate of development in barley (Hordeum vulgare). Australian Journal of Plant Physiology 23, 489–496.
| Constant and interchanged photoperiod effects on the rate of development in barley (Hordeum vulgare).Crossref | GoogleScholarGoogle Scholar |
Kernich GC, Halloran GM, Flood RG (1997) Variation in duration of pre-anthesis phases of development in barley (Hordeum vulgare). Australian Journal of Agricultural Research 48, 59–66.
| Variation in duration of pre-anthesis phases of development in barley (Hordeum vulgare).Crossref | GoogleScholarGoogle Scholar |
Kirby EJM (1988) Analysis of leaf, stem and ear growth in wheat from terminal spikelet stage to anthesis. Field Crops Research 18, 127–140.
| Analysis of leaf, stem and ear growth in wheat from terminal spikelet stage to anthesis.Crossref | GoogleScholarGoogle Scholar |
Kirby E, Appleyard M (1987) ‘Cereal development guide.’ (NAC Cereal Unit: Stoneleigh, UK)
Kirby EJM, Siddique KHM, Perry MW, Kaesehagen D, Stern WR (1989) Variation in spikelet initiation and ear development of old and modern Australian wheat varieties. Field Crops Research 20, 113–128.
| Variation in spikelet initiation and ear development of old and modern Australian wheat varieties.Crossref | GoogleScholarGoogle Scholar |
Kiss T, Balla K, Veisz O, Karsai I (2011) Elaboration of a non-destructive methodology for establishing plant developmental patterns in cereals. Acta Agronomica Hungarica 59, 293–301.
| Elaboration of a non-destructive methodology for establishing plant developmental patterns in cereals.Crossref | GoogleScholarGoogle Scholar |
Kitchen BM, Rasmusson DC (1983) Duration and inheritance of leaf initiation spike initiation and spike growth in barley. Crop Science 23, 939–943.
| Duration and inheritance of leaf initiation spike initiation and spike growth in barley.Crossref | GoogleScholarGoogle Scholar |
Miralles DJ, Slafer GA (2007) Paper presented at international workshop on increasing wheat yield potential, CIMMYT, Obregon, Mexico, 20–24 March 2006. Sink limitations to yield in wheat: how could it be reduced? The Journal of Agricultural Science 145, 139–149.
| Paper presented at international workshop on increasing wheat yield potential, CIMMYT, Obregon, Mexico, 20–24 March 2006. Sink limitations to yield in wheat: how could it be reduced?Crossref | GoogleScholarGoogle Scholar |
Miralles DJ, Richards RA, Slafer GA (2000) Duration of the stem elongation period influences the number of fertile florets in wheat and barley. Australian Journal of Plant Physiology 27, 931–940.
Riggs TJ, Kirby EJM (1978) Developmental consequences of two-row and six-row ear type in spring barley. 1. Genetical analysis and comparison of mature plant characters. The Journal of Agricultural Science 91, 199–205.
| Developmental consequences of two-row and six-row ear type in spring barley. 1. Genetical analysis and comparison of mature plant characters.Crossref | GoogleScholarGoogle Scholar |
Sanchez-Garcia M, Royo C, Aparicio N, Martin-Sanchez JA, Alvaro F (2013) Genetic improvement of bread wheat yield and associated traits in Spain during the 20th century. The Journal of Agricultural Science 151, 105–118.
| Genetic improvement of bread wheat yield and associated traits in Spain during the 20th century.Crossref | GoogleScholarGoogle Scholar | 23365469PubMed |
Slafer GA, Rawson HM (1994) Sensitivity of wheat phasic development to major environmental factors – a re-examination of some assumptions made by physiologists and modelers. Australian Journal of Plant Physiology 21, 393–426.
| Sensitivity of wheat phasic development to major environmental factors – a re-examination of some assumptions made by physiologists and modelers.Crossref | GoogleScholarGoogle Scholar |
Sreenivasulu N, Schnurbusch T (2012) A genetic playground for enhancing grain number in cereals. Trends in Plant Science 17, 91–101.
| A genetic playground for enhancing grain number in cereals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitFOmu7c%3D&md5=82f30d88c07870442ef9a71d0a9160c1CAS | 22197176PubMed |
Waddington SR, Cartwright PM, Wall PC (1983) A quantitative scale of spike initial and pistil development in barley and wheat. Annals of Botany 51, 119–130.
Whingwiri EE, Stern WR (1982) Floret survival in wheat – significance of the time of floret initiation relative to terminal spikelet formation. The Journal of Agricultural Science 98, 257–268.
| Floret survival in wheat – significance of the time of floret initiation relative to terminal spikelet formation.Crossref | GoogleScholarGoogle Scholar |
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Research 14, 415–421.
| A decimal code for the growth stages of cereals.Crossref | GoogleScholarGoogle Scholar |