Manganese toxicity in two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca) seedlings as affected by phosphorus supply
Tanja Dučić A and Andrea Polle A BA Institut für Forstbotanik, Büsgenweg 2, Georg-August Universität, 37077 Göttingen, Germany.
B Corresponding author. Email: apolle@gwdg.de
Functional Plant Biology 34(1) 31-40 https://doi.org/10.1071/FP06157
Submitted: 26 June 2006 Accepted: 24 October 2006 Published: 19 January 2006
Abstract
Manganese (Mn) is an essential micronutrient in all organisms but may become toxic when present in excess. To investigate whether interior and coastal varieties of Douglas fir [Pseudotsuga menziesii (Mirbel) Franco, var. glauca (DFG) and var. viridis (DFV)] differed in Mn tolerance, seedlings were exposed to excess Mn in hydroponic solutions. Root growth, biomass production, Mn concentrations in different tissues and Mn subcellular localisation were determined. Both varieties showed similar whole-plant Mn accumulation and biomass reduction in response to increases in Mn in the nutrient solution. Since excess Mn inhibited root elongation growth more strongly in DFG than in DFV, biomass allocation in DFV was shifted towards a relative increase in root biomass and a relative decrease in DFG. X-ray microanalysis showed that Mn enrichment in cell walls and vacuoles of DFV root cortex and epidermis cells was higher than in DFG. In roots, precipitates were observed in which Mn concentrations correlated with phosphorus (P) and to a minor extent with calcium. We suggest that the higher persistence of root growth under Mn stress in DFV was caused by greater entrapment in the vacuole and in the apoplast and by more efficient Mn detoxification in insoluble complexes with P than in DFG. To investigate whether P supply affected Mn uptake and toxicity, Douglas fir seedlings were exposed to excess Mn under P deficiency. In P-limited seedlings of both varieties, roots growth was less sensitive to excess Mn than under sufficient P-supply, although in planta Mn concentrations were not diminished. In DFG, which maintained P homeostasis under limited P supply, the negative effect of Mn stress was partially reversed, showing that Mn susceptibility is affected by P metabolism.
Additional keywords: conifer, forest decline, neophytes, nutrition, phosphorus deficiency, stress.
Acknowledgements
This work was funded by the German Science Foundation (Po362/14). We are grateful to T Riemekasten for excellent technical assistance. We thank Dr E Fritz for introduction to energy dispersive X-ray transmission electron microscopy.
Baronius K, Fiedler HJ
(1996) Nutrition status of Douglas fir (Pseudotsuga menziesii [Mirb] Franco) from Danish and German sites in comparison with the area of origin. Forstwissenschaftliches Centralblatt 115, 10–16.

Clark RB
(1982) Nutrient solution growth of sorghum and corn in mineral-nutrition studies. Journal of Plant Nutrition 5, 1039–1057.

Drew MC, Saker LR
(1984) Uptake and long distance transport of phosphate, potassium and chloride in relation to internal ion concentrations in barley: evidence of non-allosteric regulation. Planta 160, 500–507.
| Crossref | GoogleScholarGoogle Scholar |

Dučić T, Polle A
(2005) Manganese and copper toxicity and detoxification in plants. Brazilian Journal of Plant Physiology 172, 115–122.

Dučić T,
Leinemann L,
Finkeldey R, Polle A
(2006) Uptake and translocation of manganese in seedlings of two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca). New Phytologist 170, 11–20.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Farcasanu IC,
Hirata D,
Tsuchiya E,
Nishiyama F, Miyakawa T
(1995) Protein phosphatase 2B of Saccharomyces cerevisiae is required for tolerance to manganese, in blocking the entry of ions into the cells. European Journal of Biochemistry 232, 712–717.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fecht-Christoffers M,
Braun HP,
Lemaitre-Guillier C,
VanDorsselaer A, Horst WJ
(2003) Effect of manganese toxicity on the proteome of the leaf apoplast in cowpea. Plant Physiology 133, 1935–1946.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Feldman C
(1974) Perchloric acid procedure for wet-ashing organics for the determination of mercury and other metals. Analytical Chemistry 46, 1606–1609.
| Crossref | GoogleScholarGoogle Scholar |

Fritz E
(1989) X-ray-microanalysis of diffusible elements in plant-cells after freeze-drying, pressure-infiltration with ether and embedding in plastic. Scanning Microscopy 3, 517–526.

Fritz E, Jentschke G
(1994) Agar standards for quantitative X-ray-microanalysis of resin-embedded plant-tissues. Journal of Microscopy 174, 47–50.

Furihata T,
Suzuki M, Sakurai H
(1992) Kinetic characterization of two phosphate uptake systems with different affinities in suspension-cultured Catharanthus roseus protoplasts. Plant & Cell Physiology 33, 1151–1157.

Gonzalez A, Lynch J
(1999) Tolerance of tropical common bean genotypes to manganese toxicity: performance under different growing conditions. Journal of Plant Nutrition 22, 511–525.

Gonzalez A,
Steffen KL, Lynch JP
(1998) Light and excess manganese. Implications for oxidative stress in common bean. Plant Physiology 118, 493–504.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Heinrichs H,
Brumsack HJ,
Loftfield N, König N
(1986) Verbessertes Druckaufschlussystem für biologische und anorganische Materialien. Zeitschrift für Pflanzenernährung und Bodenkunde 149, 350–353.

Horiguchi T
(1987) Mechanism of manganese toxicity and tolerance of plants. 2. Deposition of oxidized manganese in plant-tissues. Soil Science and Plant Nutrition 33, 595–606.

Horst WJ
(1983) Factors responsible for genotypic manganese tolerance in cowpea (Vigna unguiculata). Plant and Soil 72, 213–218.
| Crossref | GoogleScholarGoogle Scholar |

Horst WJ, Marschner H
(1978) Symptoms of manganese toxicity in beans (Phaseolus vulgaris L.). Zeitschrift für Pflanzenernährung und Bodenkunde 141, 129–142.

Kihn JC,
Masy CL, Mestdagh MM
(1988) Yeast flocculation: competition between nonspecific repulsion and specific bonding in cell adhesion. Canadian Journal of Microbiology 34, 773–778.
| PubMed |

Kitao M,
Lei TT, Koike T
(1997) Comparison of photosynthetic responses to manganese toxicity of deciduous broad-leaved trees in northern Japan. Environmental Pollution 97, 113–118.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kitao M,
Lei TT,
Nakamura T, Koike T
(2001) Manganese toxicity as indicated by visible foliar symptoms of Japanese white birch (Betula platyphylla var. japonica). Environmental Pollution 111, 89–94.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kuo S, Mikkelsen DS
(1980) Kinetics of zinc desorption from soils. Plant and Soil 56, 355–364.
| Crossref | GoogleScholarGoogle Scholar |

Le Mare PH
(1977) Experiments on effects of phosphorus on the manganese nutrition of plants. II. Interactions of phosphorus, calcium and manganese in cotton grown with nutrient solutions. Plant and Soil 47, 607–620.
| Crossref | GoogleScholarGoogle Scholar |

Leinemann L
(1996) Genetic differentiation of damaged and healthy Douglas fir stands in Rheinland-Pfalz with respect to their origin. Silvae Genetica 45, 250–256.

Marschner H
(1986) Areas where future research on uptake and translocation of iron should be focused. Journal of Plant Nutrition 9, 1071–1076.

McPharlin J, Bieleski R
(1987) Phosphate uptake by Spirodela and Lemna during early phosphate deficiency. Australian Journal of Plant Physiology 14, 561–572.

Memon AR,
Chino M, Yatazawa M
(1981) Micro-distribution of aluminum and manganese in the tea leaf tissues as revealed by X-ray microanalyzer. Communications in Soil Science and Plant Analysis 12, 441–452.

Muchhal US, Raghothama KG
(1999) Transcriptional regulation of plant phosphate transporters. Proceedings of the National Academy of Sciences USA 96, 5868–5872.
| Crossref | GoogleScholarGoogle Scholar |

Nogueira MA,
Magalhaes GC, Cardoso EJBN
(2004) Manganese toxicity in mycorrhizal and phosphorus fertilized soybean plants. Journal of Plant Nutrition 27, 141–156.
| Crossref | GoogleScholarGoogle Scholar |

Palaniyandi R, Smith CB
(1979) Effect of nitrogen-sources on growth-responses and magnesium and manganese leaf concentrations of snap beans. Communications in Soil Science and Plant Analysis 10, 869–881.

Pfeffer PE,
Tu S-I,
Gerasimowicz WV, Cavanaugh JR
(1986) In vivo 31P NMR studies of corn root tissue and its uptake of toxic metals. Plant Physiology 80, 77–84.
| PubMed |

Quiquampoix H,
Loughman BC, Ratcliffe RG
(1993) A 31P-NMR study of the uptake and compartmentation of manganese by maize roots. Journal of Experimental Botany 44, 1819–1827.
| Crossref |

Raghothama KG
(1999) Phosphate acquisition. Annual Review of Plant Physiology and Plant Molecular Biology 50, 665–693.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Roby C,
Bligny R,
Douce R,
Tu SI, Pfeffer PE
(1988) Facilitated transport of Mn2+ in sycamore (Acer pseudoplatanus) cells and excised maize root tips. A comparative 31P NMR study in vivo. The Biochemical Journal 252, 401–410.
| PubMed |

Rout GR,
Samantaray S, Das P
(2001) Studies on differential manganese tolerance of mung bean and rice genotypes in hydroponic culture. Agronomie 21, 725–733.
| Crossref | GoogleScholarGoogle Scholar |

Sarkar D,
Pandey SK,
Sud KC, Chanemougasoundharam A
(2004) In vitro characterization of manganese toxicity in relation to phosphorus nutrition in potato (Solanum tuberosum L.). Plant Science 167, 977–986.
| Crossref | GoogleScholarGoogle Scholar |

Schöne D
(1991) Site and acid rain induced nutritional disorders of Douglas fir in southwestern Germany. Allgemeine Forst und Jagdzeitung 163, 53–59.

Schöne D
(1992) Hypothesis and observations on manganese toxicity and trace-element nutrition of Douglas fir in southwestern Germany. Allgemeine Forst und Jagdzeitung 163, 88–93.

Shimogawara K, Usuda H
(1995) Uptake of inorganic phosphate by suspension cultured tobacco cells: kinetics and regulation by Pi starvation. Plant & Cell Physiology 36, 341–351.

St Clair SB, Lynch JP
(2004) Photosynthetic and antioxidant enzyme responses of sugar maple and red maple seedlings to excess manganese in contrasting light environments. Functional Plant Biology 31, 1005–1014.
| Crossref | GoogleScholarGoogle Scholar |

St Clair SB, Lynch JP
(2005) Element accumulation patterns of deciduous and evergreen tree seedlings on acid soils: implications for sensitivity to manganese toxicity. Tree Physiology 25, 85–92.
| PubMed |

Wissemeier AH, Horst WJ
(1992) Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata (L.) Walp.). Plant and Soil 143, 299–309.
| Crossref | GoogleScholarGoogle Scholar |

Zasoski RJ,
Porada HJ,
Ryan PJ,
Greenleaf-Jenkins J, Gessel SP
(1990) Observations of copper, zinc, iron and manganese status in western Washington forests. Forest Ecology and Management 37, 7–25.
| Crossref | GoogleScholarGoogle Scholar |

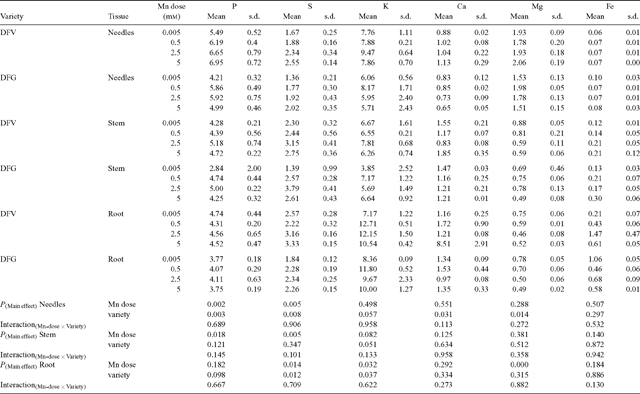
Appendix 1
|


