Cryo-scanning electron microscopy (CSEM) in the advancement of functional plant biology: energy dispersive X-ray microanalysis (CEDX) applications 1
Margaret E. McCully A D , Martin J. Canny B , Cheng X. Huang C , Celia Miller A and Frank Brink CA Division of Plant Industry, CSIRO, Canberra, ACT 2601, Australia.
B Plant Science Division, Research School of Biology, RN Robertson Building, The Australian National University, Canberra, ACT 0200, Australia.
C Centre for Advanced Microscopy, The Australian National University, Canberra, ACT 0200, Australia.
D Corresponding author. Email: margaret.mccully@csiro.au
Functional Plant Biology 37(11) 1011-1040 https://doi.org/10.1071/FP10095
Submitted: 28 April 2010 Accepted: 17 August 2010 Published: 22 October 2010
Abstract
The capacity to make measurements of elemental concentrations at the level of single cells by energy dispersive X-ray microanalysis of cryo-fixed, inherently-hydrated plant parts (CEDX) is changing or extending our understanding of many plant functions. We include in this review a wide-ranging catalogue of studies that have used CEDX which provides access to the literature on elements measured, plants and tissues studied, techniques used, level of quantitation and the significant findings. These findings include new perspectives on the following areas: salt tolerance; xylem maturation and solute content, root pressure and embolism refilling; the contents of intercellular spaces; sequestration of toxic elements; biomineralisation with silicon; movement of tracer homologues of native cations; indirect localisation of molecules with a distinctive element component; transfer of nutrients from vesicular-arbuscular (VA) mycorrhizas; the role of mucilages in protection and in generating mechanical force. In an Appendix we discuss the procedures involved in CEDX: cryo-fixation, specimen planing, etching, elemental quantitation and mapping. Limitations on sample numbers, elements measurable, spatial resolution, sensitivity and threshold concentrations quantifiable are outlined. A brief discussion of the potential of emerging technologies for cell-specific analysis of cryo-fixed, hydrated specimens is included. In the Accessory Publication we list our standard protocol for CEDX.
Additional keywords: biomineralisation, cryo-etching, cryo-fixation, cryo-planing, EDX-quantitation, elemental tracers, glucosinolates, intercellular spaces, mucilages, mycorrhizas, salt tolerance, silicon, specimen coating, toxic elements, X-ray microanalysis, xylem function.
Andersen PC,
Brodbeck BV, Mizell RF
(1992) Feeding by the leafhopper, Homalodisca coagulata, in relation to xylem fluid chemistry and tension. Journal of Insect Physiology 38, 611–622.
| Crossref | GoogleScholarGoogle Scholar |
concluded that if the ice crystals did not extend beyond the compartment in which they formed, then the elements in that compartment could be reliably quantified by adjusting the raster size (area over which X-rays are excited and collected) to cover the area of the compartment and thus obtain mean counts of the elements present, even though their distribution was heterogeneous as a result of the freezing eutectics. Markhart and Läuchli (1982) indirectly confirmed this view by showing that in barley roots frozen in different cryogens (LN2, nitrogen slush, or Freon cooled with LN2) there was no difference in the ion content measured (in vacuoles) despite the different freezing rates and consequent different ice crystal size, except that Freon introduced Cl as a contaminant.
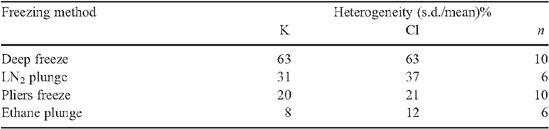
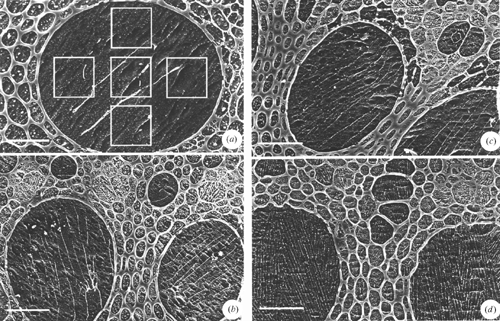
Results of more recent study of the effect on solute quantification of different ice-crystal sizes produced by different cryogens are given in Tables A1 and A2, and McCully et al. (2000). Root xylem vessels perfused with a 100 mM KCl solution were cryo-fixed in different ways, and ice crystal size revealed by the spacing of the solute lines with CSEM (Fig. A1). Crystals were very large in roots frozen in a domestic freezer at −20°C (Fig. A1a ), but with the most common (and slowest) cryogen LN2, crystal size was not seriously larger than with the much faster freezing liquid ethane (cf. Fig. A1b, d ). Crystals after freezing with cryo-pliers (Fig. S1 available in the Accessory Publication) cooled with LN2 were of intermediate size (Fig. A1c ).
|
|
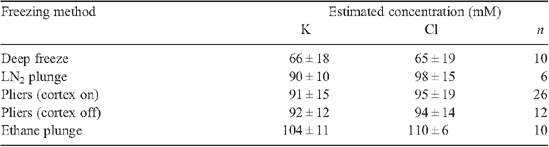
Analyses comparing mean concentrations from analyses of the KCl-perfused vessels using small rasters covering different portions of the vessel face (Fig. A1a ) with those from a single raster that included most of the vessel surface showed no significant difference in either [K] or [Cl] even in the roots frozen at −20°C (McCully et al. 2000). For example, the [K] measured in the central square of the specimen in Fig. A1a was 103 mM, in the other squares, clockwise from the bottom, 70, 53, 9.5 and 35 mM, respectively, (mean of the five estimates = 58 mM). The single measurement using the large raster gave [K] = 54 mM. The percent heterogeneity of small raster measurements, (s.d./mean)% for all similarly frozen samples was 63% for both [K] and [Cl]. The slowly frozen sample (Fig. A1a ) is clearly unsatisfactory for quantitative analyses since almost 50% of the solute content was lost during freezing. Samples frozen with LN2 (Fig. A1b ), cryo-pliers cooled in LN2 (Fig. A1c ), or ethane (Fig. A1d ) had decreasing heterogeneity of small raster measurements in that order. There was no significant deviation from the concentration of the perfused liquid in measurements made using a large raster for any of these three freezing methods (Tables A1 and A2).
|
Long distance transport of cryo-fixed specimens (including overseas) can be in a commercially available ‘dry shipper’. These shippers, when correctly filled, contain no free LN2, which is all absorbed in a solid matrix. LN2 temperatures can be maintained for several weeks. Regulations governing which shippers are allowed on aircraft or accepted by courier companies vary – so check before purchasing. We occasionally freeze fragile or easily dislodged material (e.g. rhizosheaths) by placing them into a cryo-vial and freezing directly in a cold dry shipper. This is also a good compromise if LN2 can’t be taken to the field.
Cryo-fixed samples can probably be stored indefinitely in a commercial cryo-storage unit. Good evidence that cryo-storage at LN2 temperature preserves the cryo-fixed state unchanged over long periods is that snowflakes cryo-fixed for examination of their morphology and geometry, kept their initial shapes without sublimation for at least 3 years in a cryo-store (Erbe et al. 2003). We have no information on the diffusivity of ions or molecules in ice at −196°C. Until it is proved otherwise, the assumption that the distribution of solutes in the ice remains unchanged seems justified.
Preparing a specimen for analysis
To observe and analyse tissues and cells it is necessary to expose their internal structure after they have been cryo-fixed. This can be done in two ways: by fracture of the frozen tissue or by cryo-planing. With both methods it is important that the new surface produced is well separated from damage done during the earlier breakage of the specimen.
The cryo-preparation chambers sold with the available cold-stages have built into them levers or knives for fracturing frozen specimens under vacuum to produce a fresh surface for observation. For some structural observations such fracture faces are adequate or superior. But as emphasised in Huang et al. (1994), Nijsse and van Aelst (1999) and McCully et al. (2000), there are many advantages in planing a smooth surface in the specimen in a controlled plane for CSEM observations. For accurate X-ray microanalysis the planed surface is absolutely necessary (Hess et al. 1975; Huang et al. 1994). Irregular surfaces with hills and valleys scatter electrons, obscure X-rays where projections come between their source and the detector, and spurious X-rays are produced and scattered from regions raised above the surface, distorting the EDX spectrum, emphasising some wavelengths and obscuring others. Goldstein et al. (2003) state that ‘rough surfaces represent the greatest challenge to quantitative X-ray microanalysis’.
Another advantage of cryo-planing over fracturing is that specimens can be replaned after observation in the SEM if carefully transferred back to the microtome in LN2. The metal coating is planed off and a new surface obtained at the desired depth. In this way multiple samples can be analysed from different depths in the same piece of tissue. Such a series of block faces is illustrated in figs 7–10 by Huang et al. (1994). These authors also showed that replaning had no significant effect on element concentrations (see their table 2). If desired, planed specimens on their stubs can be stored for future use in cryo-vials in LN2 or a cryo-store.
Note: although researchers have occasionally used hydrated cryo-sections for EDX analysis, we do not recommend this. Although such sections potentially yield higher detection sensitivity than bulk frozen material, it is very difficult to produce adequate sections of plant tissue (Frey 2007) because of the heterogeneous rigidity of cell walls, frozen cell contents and empty spaces. Indeed, Walther and Müller (1999) have shown that cryo-planed block faces are more stable and yield better resolution of tissue details than cryo-sections.
Etching
The planed frozen surfaces of plant tissues have no visible tissue structure except empty spaces (fig. 2 by Huang et al. 1994), and to reveal the cells to be measured it is necessary to remove a very thin layer of ice (nm). This is called etching, and is another important requisite for accurate quantitative analysis of plant tissues. Etching, or sublimation, is done by putting the cryo-planed specimen on the microscope cold stage (−160°C), forming an image at low voltage (~1 kV), and raising the stage temperature slowly to approximately −90°C, to sublime any frost that formed during specimen transfer and a small amount of the surface ice.
Plant tissue has the advantage over animal tissue in that the cells are surrounded by cell walls, which show up immediately in the surface when a little ice is removed, permitting selection of the required sites. Because they lack cell walls, similar monitoring of etching for animal tissues is difficult, and Marshall and Xu (1998) recommend a different approach for locating regions of interest and accurate quantitation of such tissues. The specimens were not etched, but structural features located by first mapping oxygen or carbon distribution. However, it is impossible to locate fine details of plant tissues with such maps (e.g. fig. 7 by Marshall and Xu 1998) so we recommend careful etching. This etching is necessary in any event to remove frost formed during specimen transfer which will interfere with the analysis.
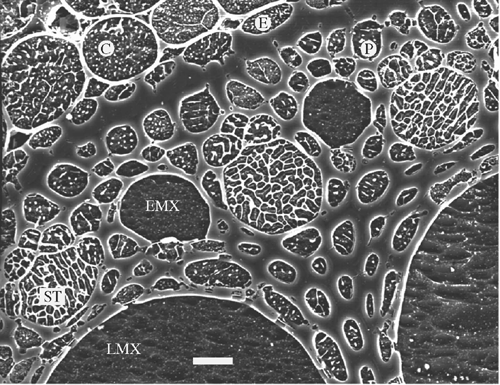
Satisfactorily etched (and Al-coated) samples are illustrated in Figs 1a, c, e–i , A1, A2, and in fig. 14.9 by Goldstein et al. (2003). Fig. A2 also shows that patterns of sequestered solutes differ in different cell types according to the nature and amounts of solute present. Here, lack of eutectic lines in the xylem vessels indicates a very low concentration of solutes in the sap, while other cells have prominent solute sequestration. The reticulate pattern in the sieve tubes is characteristic of their high sugar content.
|
Huang et al. (1994) have shown (their table 1) that with minimal etching at −90°C of cryo-planed carbon slurry standards for Cl, K and P, percentage ratios determined for these elements were not different from those of unetched standards, but significantly higher for the same standards etched at −80°C.
Some papers report the use of a standard etching time at a given temperature, but this does not accommodate the inherent variation within and between specimens. Prolonged etching as a technique to increase the count rates and the sensitivity of the element detection may help qualitative studies, but accurate quantitation of elements in the resulting partially freeze-dried specimens is impossible, not only because different elements sublime at different rates, but also they appear more concentrated because of the change in matrix composition and increase in the size of the X-ray analytical volume (see table 14.5 and fig. 14.13 by Goldstein et al. 2003).
Coating
The low kV used for watching loss of ice while preventing the build-up of charge on the specimen has not sufficient energy to excite all the elements of interest to emit X-rays. At the necessary higher kVs internal and surface charge is likely to build up in the specimen (e.g. fig. 15.4 by Goldstein et al. 2003), which interferes with both observation and analysis. Coating the specimen with a thin layer of conducting material will disperse the built-up charge. A variety of elements have been evaporated or sputtered on specimens for this purpose (Table 1), but for quantitative CEDX only three elements, Al, Cr and C are possible (Marshall 1998; Goldstein et al. 2003). Other metal coatings (e.g. Au, Ni, Pt), which are excellent for CSEM structural observations, are not suitable for quantitative analysis because their coating requires a temperature high enough to vaporise some of the elements to be measured, and they interfere differentially with X-ray emission from different elements. Of the three elements suitable for coating for CEDX work, Al has the most desirable features (tables 1 and 2 by Marshall 1998; table 15.1 of Goldstein et al. 2003) and we routinely use it (Figs 1a, c, e–i , A1, A2). Different thickness of coating with Al has been shown not to affect the elemental counts obtained, provided the counts are collected for a standard live time (Reid et al. 1993). That paper corrected an earlier one from the same group (Hopkins et al. 1991), which reported (wrongly) that counts varied with Al coat thickness. Therefore, variations in Al thickness over a specimen are of little consequence.
The sampling problem
Obtaining a meaningful number of replicate analyses is a challenge because of the time required for each one, and the expense for instrument time. The current generation of EDX analysers are considerably faster than older models, easing the problem somewhat, and the new silicon drift detectors (Newbury 2006) will further reduce spectrum collection time. It is important that for each study there must be similar organs from several plants represented, and samples of each of the cell types in the specific region of interest in each organ. The operator must judge, from the peak counts recorded and their variance, how many cells, organs and plants should be measured for the information required. Bear in mind that, for a Gaussian distribution, the variance is reduced as the square of the number of observations. So that if, after five estimates the variance is 20% of the mean, and you would like it to be 10%, you must do four times as many estimates (20). Consider whether the improvement is worth the time and money expended. Such judgements become critically necessary when trying to confirm differences between cells or cell types.
Quantitation
Conversion of primary X-ray analytical data from biological specimens to element quantitative data requires comparison with the same data from standards of known elemental composition. These standards must resemble the specimens as closely as possible chemically, be prepared for analysis identically, and be analysed with the same instrument conditions. The review by Warley (1990) discusses these requirements in detail. Although she deals with standards which have proteins added to aqueous solutions of elements to mimic animal cells, the principles outlined are applicable also to frozen, hydrated plant cells. Although protein-containing standards may be good for protein-rich plant meristem cells, there have been several approaches to making standards more appropriate for the water- and carbon-rich environment of cryo-fixed, hydrated plant tissues, particularly cell vacuoles and apoplastic liquids. Cell wall ghosts of killed tissue, cotton wool, carbon filters or xylem vessels loaded with solutions of inorganic solutes of known composition have all been used (Table 1), but test solutions containing colloidal graphite are most adaptable (Treeby et al. 1987; Van Steveninck and Van Steveninck 1991).
Achieving the most useful information from CEDX
All the studies documented in this review have in some measure advanced knowledge of plant function. As the CEDX technique has developed to yield both qualitative and more quantitative data, the usefulness of the information obtained has greatly increased. Now the most important potential of the technique for plant biologists is for accurate quantification of elements at the tissue/cell specific level in fully functioning plants.
Accurate quantification requires (1) cryo-fixation; (2) smooth, cryo-planed surfaces coated with Al, Cr or C for analysis; (3) appropriate settings on an SEM with high beam stability; (4) analysis of specimens in their inherent state of hydration; and (5) quantification of spectral data by use of frozen standards that mimic the properties of the specimens. (These conditions were most closely matched in studies indicated by QD in text Table 1.) An illustration of a specimen prepared according to these criteria, and showing a variety of cells that could be analysed, is given in Fig. A2.
Specimens that most closely retain the elemental distributions in the functioning plant should, ideally, be cryo-fixed in situ in undisturbed plants. The artefacts introduced by dissecting out small pieces for freezing can outweigh those produced by the non-vitreous freezing of selected regions.
Mapping
A visual image of the distribution of an element in a specimen observed by the SEM (a map) can be made by scanning the electron beam across the specimen in a raster of lines and recording the arrival of an X-ray at the detector as a flash of light on the fluorescent screen that displays the field of view. The sum of the flashes building up over time is recorded by a camera with its shutter kept open. These maps are called dot-maps, and are the only maps found in the early literature. Examples are shown in Fig. 1f–h . With the development of computer-controlled digital imaging that replaced photographic imaging, it became possible to generate digital maps by recording the X-ray spectrum from each pixel where the beam remained fixed for a standard dwell time (e.g. 100 s). Dot maps could record only one element at a time. Digital maps contain data on all the elements recorded in each pixel, and can be mined to extract images of any element present (Friel and Lyman 2006). Digital maps have now replaced the dot maps. Either kind of map is a most useful and informative representation of which elements are in which parts of the specimen. They are particularly useful as an initial survey to direct choices of where to concentrate sampling for quantitative measurements.
At present, it is not feasible to make quantitative measurements of elemental concentrations from the maps produced by CEDX, because of the long time required to collect the data (hours). Over such times, specimen drift, contamination and electron beam damage distort the information in the map. Also, the supply of LN2 to keep the stage cold requires special arrangements. (Marshall and Xu 1998; fitted large LN2 containers on their microscope to hold the necessary coolant for overnight analyses.) It is hoped that with the new generation of silicon drift X-ray detectors (Newbury 2006), quantitation of X-ray maps may become feasible with CEDX.
Limitations on CEDX
The fact emphasised repeatedly in this review – that the specimens remain in the state of hydration prevailing when they were cryo-fixed – though a major advantage of CEDX, has the consequence that the presence of so much water interferes in several ways with the analysis. The ice matrix in which the elements are embedded restricts access of the accelerated electrons to a small depth (~2 µm). The ice also retards to some extent the X-rays which are the basis of measurement. The consequence is a limit on the threshold of detection of all the elements. The lighter the element, the weaker are the emitted X-rays, and the higher is the threshold of detection. That threshold is the size of a peak that can be reliably distinguished from the background Bremsstrahlung. Those elements most affected are carbon, nitrogen and sodium. Even providing the detector with an ultra-thin window in place of the normal Be window, or omitting the window altogether (windowless), leaves these elements difficult to quantify.
So a major parameter in the calibration of an element is the lowest reliable concentration that can be measured (distinguished from background). Our approach to measuring the threshold is to identify the standard deviation of the calibration measurements at the low concentration end of the series. Then use Student’s t-test to decide where on the calibration curve a concentration becomes significantly different from a mean of zero with the same standard deviation. For [K] this is ~10 mM, for Na, as high as ~40 mM. Using CEDX, one must balance this disadvantage against the other advantages. For trace elements whose concentration is far below 1 mM, some other technique is necessary.
The surrounding water matrix has another negative influence on detection and quantification of the light elements, namely the very large size of the O Kα peak, and the presence of the O Kβ peak close by. The O Kα peak completely obscures the N peak and renders that important element undetectable and unquantifiable. The impossibility of measuring NO3 –, NH2 +, NH3 and amino acids in a cryo-fixed legume nodule is a matter for chronic regret.
The other elements interfered with by O, C and Na, can still be quantified, but only if present in substantial concentrations. The Na Kα peak coincides with the O Kβ peak, and it is necessary to correct the Na reading by subtraction of a proportion of the O Kα peak, which may be measured in Na-free standard solution. A similar correction to Ca Kα peak needs to be applied for the overlap with the K Kβ peak (Lazof and Läuchli 1991a ).
The water matrix limits also the spatial resolution achievable in cryo-fixed plant parts. As explained above, pieces of plant of any useful size freeze slow enough to contain quite large ice crystals (Fig. A1). This, combined with the very small volumes of many plant protoplasts, often restricts measurements to vacuoles. Cell walls also can prove unanalysable. Because, fully-hydrated, they contain ~70% C, the incident electron beam can cause so much heating of their small volume that they are seen to be torn apart. What the X-ray signal from such a process signifies is unclear.
Another limitation has been mentioned above, namely the long collection times at present needed for constructing digital maps. As was said there, this negates the reliable quantification of maps, but the Si-drift detectors may remove this limitation.
In spite of all these limitations, much important information about functional plant biology has been obtained by CEDX, as witness the text of this review and Table 1. As has been stressed also, there are many important new questions that await investigation by CEDX.
Emerging technologies
There are four other analytical technologies for locating elements in plant tissue which are being developed, and which may supplement CEDX. At present none of them yields fully quantitative measures of the elements, and only recently are some being adapted to take advantage of the reliability of hydrated cryo-fixed tissues. Their main advantages and limitations are outlined.
Combining the EDX analyser with an environmental scanning electron microscope (EDX-ESEM) has the advantage that the specimen is held at low vacuum (up to 2 Torr), so that specimen preparation is reduced to a minimum. EDX-ESEM is largely limited to the study of surfaces of intact specimens. With cut faces of organs, only insoluble elements are reliably retained. It is at best semiquantitative. An assessment of its advantages and disadvantages is given by Egerton-Warburton et al. (1993) and Griffin and Suvorova (2003). Recent uses of it are by Lux et al. (2003) for locating Si in bamboo tissue, and by Harada and Choi (2008) for revealing Cd in exudate from tobacco glandular hairs.
Combining EDX with proton induced X-ray emission (EDX-PIXE) substitutes a beam of protons for the electron beam of CEDX, and has the advantage that the X-ray peaks have a negligible background. This makes the detection limits very much lower than with EDX-SEM (down to 1 µg g–1 for Mn, Fe, Ni, Cu, Zn). It does not provide clear morphological images of the specimens to locate the target areas. It has mainly been used with dehydrated tissue, but has been adapted recently for cryo-fixed hydrated specimens by Tylko et al. (2007).
Combining EDX with electron energy loss spectroscopy (EELS) locates and quantifies elements in insoluble compounds in dehydrated material. It is valuable for revealing the composition of crystals in plant tissues, and was used by Lichtenberger and Neumann (1997) for confirming the identity of crystals of Ca oxalate, SiO2 deposits, Zn-silicates in cell walls, and Cu-chelates in idioblasts.
Secondary ion mass spectrometry (SIMS) uses a beam of primary ions (+ or –) to sputter secondary ions from the surface of a sample. These may be cations or anions or neutral, and are sorted by the mass spectrometer according to their mass. This reveals the nature and amount of the element that produced them. The great advantage of this technique is that it can analyse elements of any atomic number (all elements and isotopes) with great sensitivity. The power to sort isotopes opens up limitless possibilities for tracer studies with exact homologues of the native elements (see ‘Tracer elements’). Stable isotopes may be used for continuous-labelling and pulse-chase studies of exchange and transport. Until very recently it was universally used with dry specimens. The use of SIMS with cryo-fixed, fully-hydrated, smooth-planed material has been pioneered by Metzner et al. (2008, 2010a , 2010b ), who have produced amazing semiquantitative images of the very fine-scale exchanges of K and Na from xylem vessels into the cell-wall apoplast of xylem parenchyma and surrounding cells. To combine the SIMS images with anatomical images it was necessary to move the specimens to a cryo-SEM. The long time for collection of the images (up to 8 h), and the awkward need to transfer the specimen between two instruments, enforce a strong limitation on the number of samples that can be analysed, but we look forward to the formulation of a whole new science of tissue/element relations.


