Medicago truncatula as a model for understanding plant interactions with other organisms, plant development and stress biology: past, present and future
Ray J. RoseAustralian Research Council Centre of Excellence for Integrative Legume Research, School of Environmental and Life Sciences, The University of Newcastle, Callaghan, NSW 2308, Australia. Email: ray.rose@newcastle.edu.au
Functional Plant Biology 35(4) 253-264 https://doi.org/10.1071/FP07297
Submitted: 17 December 2007 Accepted: 16 April 2008 Published: 3 June 2008
Abstract
Medicago truncatula Gaertn. cv. Jemalong, a pasture species used in Australian agriculture, was first proposed as a model legume in 1990. Since that time M. truncatula, along with Lotus japonicus (Regal) Larsen, has contributed to major advances in understanding rhizobia Nod factor perception and the signalling pathway involved in nodule formation. Research using M. truncatula as a model has expanded beyond nodulation and the allied mycorrhizal research to investigate interactions with insect pests, plant pathogens and nematodes. In addition to biotic stresses the genetic mechanisms to ameliorate abiotic stresses such as salinity and drought are being investigated. Furthermore, M. truncatula is being used to increase understanding of plant development and cellular differentiation, with nodule differentiation providing a different perspective to organogenesis and meristem biology. This legume plant represents one of the major evolutionary success stories of plant adaptation to its environment, and it is particularly in understanding the capacity to integrate biotic and abiotic plant responses with plant growth and development that M. truncatula has an important role to play. The expanding genomic and genetic toolkit available with M. truncatula provides many opportunities for integrative biological research with a plant which is both a model for functional genomics and important in agricultural sustainability.
Additional keywords: abiotic stress, biotic stress, Jemalong 2HA, legumes, nodulation, regeneration.
Introduction
Medicago truncatula Gaertn. (barrel medic) is now well accepted as a model legume species, and, together with Lotus japonicus (Regal) Larsen, has helped to bring legumes into the position of being able to access many of the contemporary tools of genetics and functional genomics. Two recent demonstrations have emphasised the success obtained by a focus on model legumes. The activation (Gleason et al. 2006; Tirichine et al. 2006) of a calcium- and calmodulin-dependent protein kinase (CCaMK), and a gain of function mutation for the cytokinin receptor (Tirichine et al. 2007) Lotus histidine kinase 1 (LHK1), can cause the spontaneous formation of nodules independent of rhizobia. These latter results have also again raised the possibility of transfer of the rhizobia–legume symbiosis into other plant species (Oldroyd 2007). In this review, I wanted to consider the background to how M. truncatula became an important contributor to functional plant biology, particularly given its historical role in Australian agriculture, as well as its potential for integrating what were once considered discrete areas of investigation. M. truncatula is more than a model for plant–microbe interactions. In this context, I wanted to also highlight work on the interaction with pests and pathogens, plant development and stress biology. To maximise its reproductive success, the sessile flowering plant has to interact effectively with its biotic and abiotic environment as it grows and develops.
Medicago truncatula cv. Jemalong, agriculture and biotechnology
Annual Medicago species (annual medics) of Mediterranean origin have been particularly important in southern Australian agriculture because of their role in the wheat/sheep rotation. Cereal crops are alternated with legume pastures. The value of M. truncatula was recognised as early as 1939 and Jemalong was a commonly used cultivar (Crawford et al. 1989). This is an excellent example of sustainability with the pasture providing forage for livestock and symbiotically fixed nitrogen becoming available for subsequent crops (Loi et al. 2000). The annual medics germinate following the softening of hard seeds set in previous years (Crawford et al. 1989). More recently, a second generation of annual pasture legumes have been introduced to cater for a wider range of farming systems including grain legumes and oil seeds in phase farming systems (Loi et al. 2005).
With the advent of somatic hybridisation (Carlson et al. 1972) and then Agrobacterium-mediated transformation in 1983 (Bevan et al. 1983; Fraley et al. 1983; Herrera-Estrella et al. 1983; Murai et al. 1983), biotechnology was directed to many agricultural species including legumes. Regeneration was a requirement for these techniques. Regeneration from cultured tissues or protoplasts was conducted with the perennial, allogamous and autotetraploid Medicago sativa L., which proved amenable to regeneration via somatic embryogenesis from tissue explants (Saunders and Bingham 1972) and protoplasts (Johnson et al. 1981; Rose et al. 1986). Regeneration was restricted to special lines, notably Regen S, which was bred for regenerability (Bingham et al. 1975). Annual Medicago species were difficult to regenerate, but work by Bingham and co-workers indicated that the Medicago genus included species with ‘regenerability’ genes. M. truncatula, an annual autogamous diploid, is more attractive for transformation work and the associated genetics than the allogamous autotraploid M. sativa. The cultivar Jemalong proved amenable to regeneration but at extremely low frequency. What was surprising was that the few plants that regenerated, when used as explants, had a huge increase in regenerability (500×) via somatic embryogenesis and this was inherited (Nolan et al. 1989) and lead to the development of the highly regenerable Jemalong 2HA (2HA) (Rose et al. 1999). Using 2HA, transformation (Thomas et al. 1992; Chabaud et al. 1996; Wang et al. 1996), regeneration from protoplasts (Rose and Nolan 1995), asymmetric somatic hybridisation (Tian and Rose 1999) and transfer of agriculturally important genes such as viral resistance genes was feasible (Jayasena et al. 2001). Use of transformation as a tool for analysing gene function became crucial as whole-genome sequencing, large scale mutant isolation and the era of functional genomics began.
Medicago truncatula as a model legume
The foremost plant model Arabidopsis thaliana (L.) Heynh has many advantages for plant genomics, with its small size, short generation time, large numbers of offspring and small nuclear genome. Sequencing commenced in 1996; and its sequence was published in December 2000 (The Arabidopsis Genome Initiative 2000). The value of Arabidopsis mutants for functional analysis was shown some years ago by the isolation of mutants of C3 species with defects in CO2 assimilation and photorespiration (Somerville and Ogren 1979). The ability to transform Arabidopsis by the floral dip method (Clough and Bent 1998) has greatly facilitated research in Arabidopsis by enabling genome-wide insertional mutagenesis with T-DNA insertions (Alonso et al. 2003) to potentially enable knockouts of all genes. Arabidopsis is a dicotyledonous but non-nitrogen-fixing plant, making it important to develop a legume model with its ability to establish symbiotic interactions with rhizobia and mycorrhizae. With more than 18 000 species, legumes are the third largest family of higher plants (Young et al. 2003) and their ability to utilise atmospheric nitrogen fixation by the rhizobia symbiosis is of key importance in the biosphere and in agricultural systems.
In 1990, Barker et al. (1990) proposed M. truncatula as a model legume for the study of the molecular genetics of the rhizobia-legume symbiosis. M. truncatula (2n = 2x = 16) is diploid and autogamous, has a relatively small genome (~500 mbp – Bennett and Leitch 1995) and a generation time of ~3 months in long day conditions (Barker et al. 1990). These latter characteristics make it a more suitable model than the allogamous and perennial autotetraploid, lucerne, but like M. sativa it is efficiently nodulated by Sinorhizobium meliloti (Barker et al. 1990). The Sinorhizobium meliloti genome has now been sequenced (Galibert et al. 2001). A key reason for the choice of Jemalong in the Barker et al. (1990) proposal was its regenerability, which is relatively rare amongst annual legumes; and their preliminary work on transformation with Agrobacterium. My laboratory initiated work with M. truncatula cv. Jemalong in 1987 as it was the only M. truncatula cultivar that showed some regenerability. The regenerability was greatly enhanced by a cycle of tissue culture (Nolan et al. 1989) and subsequent selection for regenerability through the seed line lead to the development of Jemalong 2HA (2HA, Rose et al. 1999). We reported the first M. truncatula transformation with leaf explants using Agrobacterium rhizogenes and the more effective Agrobacterium tumefaciens procedure (Thomas et al. 1992). Subsequently we published a procedure using Agrobacterium tumefaciens (Wang et al. 1996). Several other transformation protocols have subsequently been published (see review Rose et al. 2003; Crane et al. 2006). Chabaud et al. (2003) have studied the kinetics of 2HA transformation, compared Agrobacterium strains and have used different reporter genes. Transformed 2HA with a single T-DNA insertion produce T1 seed that segregate in a 3 : 1 ratio (Wang et al. 1996; Chabaud et al. 2003).
Though plant transformation via somatic embryogenesis is very useful for RNAi and overexpression studies for functional genomics, it is still a relatively long process of 4–5 months (Chabaud et al. 2003), and really high throughput transformation comparable to Arabidopsis remains a difficulty. The 2HA Jemalong line is, nevertheless, valuable for routine transformation from leaf explants (Chabaud et al. 2007), molecular breeding (Jayasena et al. 2001), understanding somatic embryogenesis mechanisms (Nolan et al. 2003) and transposon mutagenesis (Ratet et al. 2006). The A17 Jemalong line which is being sequenced (Kulikova et al. 2001; Young et al. 2005) does not regenerate via somatic embryogenesis but can be transformed by organogenesis procedures (Trieu and Harrison 1996; Zhou et al. 2004). The recent Zhou et al. (2004) procedure requires dissecting out cotyledonary nodes from emerging seedlings and rigorous selection procedures to avoid untransformed shoots (Zhou et al. 2004).
What has been a very valuable transformation adjunct in M. truncatula and the study of the rhizobia and the arbuscular mycorrhizal (AM) symbiosis has been the use of Agrobacterium rhizogenes to form transformed roots on composite plants (Boisson-Dernier et al. 2001). This protocol involves the inoculation of sectioned seedling radicles which form the hairy roots of the composite plants. Antibiotics such as kanamycin can be used to select for co-transformation of hairy roots with introduced constructs. The hairy roots successfully form nodules after inoculation with Sinorhizobium meliloti and can be colonised by AM fungi. The transgenic roots can be generated rapidly in 2–3 weeks which has been a great aid to M. truncatula symbiosis research.
Medicago truncatula was a focus for several meetings and workshops in the United States and Europe in the 1990s to establish it as a model and initiate the development of the necessary genetic and genomic tools (Cook et al. 1997; Cook 1999). Expressed sequence tags (ESTs) were rapidly developed, beginning with the M. truncatula root hair enriched cDNA library (Covitz et al. 1998) producing 899 ESTs. There are now 227 000 M. truncatula ESTs on the The Gene Index Project database (http://compbio.dfci.harvard.edu/tgi/, accessed 15 December 2007). The first steps towards sequencing were taken when Nam et al. (1999) produced the first BAC clones from Jemalong A17. The first published genetic map of M. truncatula was produced by Thoquet et al. (2002) using two homozygous lines selected from Jemalong (Jemalong 6 or J6) and the Algerian natural population DZA315. Thoquet et al. (2002) noted that the three Jemalong lines A17, J5 and J6 could be considered to have an identical genotype (but different to the highly regenerable Jemalong genotype 2HA). BAC clones were mapped to M. truncatula A17 pachytene chromosomes by fluorescent in situ hybridisation (FISH), (Kulikova et al. 2001; Choi et al. 2004a; Kulikova et al. 2004). In the Choi et al. (2004a) study the mapping population was derived from A17 × A20. The A20 genotype is an M. truncatula ecotype with nodulation characteristics similar to A17 and a dominant leaf spot phenotype (Penmetsa and Cook 2000). From this work, it was inferred that gene-rich regions were located in the euchromatin rich chromosome arms, with the heterochromatin located in the centromere and pericentromeric regions This meant that if BAC clones could be identified as gene rich then most of the genespace could be obtained by BAC-by-BAC sequencing (Young et al. 2005). This latter strategy of anchored, clone-by-clone sequencing (as opposed to whole-genome shotgun sequencing) has been pursued. Genome sequencing began in 2002 (Young et al. 2005) and has continued until the present time (http://www.medicago.org/genome/, accessed 15 December 2007). In the M. truncatula genome assembly version 1.0 (Mt1.0) nearly 2000 BACs have been sequenced representing ~186.2 Mbp of non-redundant genome sequence, about two-thirds of the gene rich space. The completed sequencing of the gene rich space is expected by the end of 2008. This latter information can be accessed on the publicly available databases (http://www.medicago.org/, accessed 15 December 2007). Physical and genetic maps are available on this latter site. The reference mapping population is A17 × A20 (Ané et al. 2008). Genome conservation between M. truncatula and crop and other legumes, including Lotus japonicus, has been examined (Choi et al. 2004b; Cannon et al. 2006). There is substantive synteny between M. truncatula and M. sativa (Thoquet et al. 2002; Choi et al. 2004a) and M. truncatula and Pisum sativum L. (Choi et al. 2004b; Aubert et al. 2006). These mapping studies will provide valuable information for fundamental research and translational research with crop legumes.
Genetic and genomic tools available in M. truncatula
As outlined above there are now large numbers of ESTs, an increasing amount of gene rich genome sequence and physical and genetic maps available for M. truncatula. The EST information (227 000 ESTs) has been assembled into 18 612 TCs (tentative consensus sequences) and 18 238 singleton ESTs (http://compbio.dfci.harvard.edu/tgi/, accessed 15 December 2007). Current estimate of gene number from the sequencing program in M. truncatula is 42 358 (http://www.medicago.org/, accessed 15 December 2007). The M. truncatula chloroplast genome, which contains only one copy of the inverted repeat, is also an active area of study (Shaver et al. 2008).
For functional genomics it is necessary to develop forward and reverse genetics tools. The first mutations affecting nodulation phenotypes were obtained in M. truncatula using γ-rays (Sagan et al. 1995) and ethylmethane sulfonate (Benaben et al. 1995; Penmetsa and Cook 1997). Mutants have been crucial to the progress in understanding the mechanism of nodulation. Several developmental mutants, other than nodulation, have also been isolated by Penmetsa and Cook (2000). Reverse genetic strategies to infer gene function based on induced variation within a specific gene sequence are also being pursued in M. truncatula. These are RNAi, TILLING (Targeting Induced Local Lesions in Genomes), Tnt1 insertional mutagenesis (Ratet et al. 2006), and a fast neutron deletion mutagenesis based system. Using Tnt1 retrotransposon-tagged mutants a leaf development gene SINGLE LEAFLET1 has been identified (Wang et al.2008). The current status of these reverse genetic strategies has recently been discussed by Ané et al. (2008). We have recently used dexamethasone inducible RNAi (Mantiri et al. 2008) to identify SOMATIC EMBRYO-RELATED FACTOR1 (MtSERF1).
Transcriptomics have been facilitated by the development of microarrays following the completion of the large scale EST projects. A spotted 16 K microarray of 70-mer oligos (http://www.noble.org/medicago/NSF/nsf.activities.html, accessed 15 December 2007) available in 2003 has been used in gene expression studies, for example in AM development (Hohnjec et al. 2006). The Affymetrix Medicago GeneChip became available in 2005 and includes 32 167 M. truncatula ESTs and 18 733 gene predictions from M. truncatula genome sequences, 1896 cDNAs from M. sativa and 8305 gene predictions from Sinorhizobium meliloti (Dandgeard) de Lajudie et al. (Tesfaye et al. 2006). Udvardi and colleagues (2007) have also developed high-throughput quantitative reverse transcriptase (qRT–PCR) analysis of transcription factors, which is more sensitive than DNA array hybridisation methods. These authors also discuss methods to identify transcription factor target genes in a non-biased, high throughput manner.
In parallel with the development of the genetic, genomic and transcriptomic resources has been the development of an increasing amount of proteomic data for M. truncatula (e.g. Mathesius et al. 2001; Watson et al. 2003; Imin et al. 2004, 2005). This latter technology relies on protein separations and mass spectrometry. Proteins separated on two dimensional gels and trypsin digested proteins can be characterised by peptide mass fingerprints by MALDI-TOF-MS and then identified from available sequence information on publicly available databases for M. truncatula (Imin et al. 2004). Using LC/MS/MS protein spots can be fragmented into peptides and sequence information generated from an individual peptide (Imin et al. 2004). High throughput LC/MS/MS is an alternative to gel separation of proteins (Millar et al. 2005).
With all the approaches now available for functional genomics there is increasing reliance on bioinformatic resources and there are recent publications that have tabulated these resources (Cannon et al. 2005; Stacey et al. 2006).
The interaction of M. truncatula with other organisms
The M. truncatula model has been central to the progress in unravelling the legume-rhizobia symbiosis but is also proving valuable in other areas. Outlined below are several areas where the M. truncatula system is proving useful in enhancing understanding of how plants interact with symbionts, pests and pathogens.
The rhizobia–legume symbiosis
The focus on the two legume models, M. truncatula and L. japonicus, has enabled substantive progress in the understanding of how Nod factors are perceived and the signalling pathway that ultimately leads to nodule formation. As the focus of this review is M. truncatula, the schematic in Fig. 1 draws attention to key M. truncatula mutants in the developing understanding of nodulation. Progress on the mechanism of nodulation has been reviewed in recent times (Riely et al. 2004; Oldroyd and Downie 2006; Stacey et al. 2006) and includes the Lotus mutants. Identifying the receptors and linking the signalling through to the Ca2+ spiking and the apparent phosphorylation of the GRAS-type NSP1 and 2 transcription factors provides a strong framework for understanding nodule development. There are many steps in Fig. 1. to complete, particularly the integration of the development of the infection thread and the setting up of the symbiosomes associated with the morphogenesis of the nodule. Development of current thinking can be seen in several recent commentaries (Cullimore and Dénarié 2003; Udvardi and Scheible 2005; Oldroyd 2007).

|
The work on understanding signalling in nodulation is not only significant for understanding the mechanism of nodulation but also facilitates understanding of the AM symbiosis as well as interaction with pests such as nematodes. Further, the M. truncatula nodule is indeterminate producing an apical meristem. This provides a useful model to study organ morphogenesis and cell differentiation (Brewin 1991).
The arbuscular mycorrhizal legume symbiosis
Medicago truncatula has proven a useful model for investigating the arbuscular mycorrhizal symbiosis with the obligate biotroph Glomus spp. (Liu et al. 2003; Hohnjec et al. 2006). The AM symbiosis association enables a carbon supply for the fungus and an enhanced supply of mineral nutrients, notably phosphorus, to the plant (Liu et al. 2003; Hohnjec et al. 2006). The AM symbiosis as for the rhizobia symbiosis has important implications for ecology and sustainable agricultural systems. Using a legume model has the advantage of comparative studies of nodulation and mycorrhisation. Studies of mutants defective in nodulation have shown mechanistic similarities between nodulation and mycorrhizal infection (Stacey et al. 2006). Several M. truncatula mutants are defective in both the rhizobia and the AM symbiosis and the impaired genes are known as SYM genes (Parniske 2004). An example of these genes are the DMI1, DMI2 and DMI3 genes (Fig. 1), involved in both Nod factor and Myc factor signalling (Ané et al. 2004; Stacey et al. 2006). Though the rhizobia and AM response have some common signalling components, the NFR1 and NFR5 Nod-factor receptors are not the AM receptors (Parniske 2004). Transcriptional profiling studies using microarrays reveal that several hundred genes are upregulated during AM development and how these genes relate to current understanding of signalling remains to be explored (Hohnjec et al. 2006).
Harrison and co-workers (Harrison et al. 2002; Javot et al. 2007) have defined a M. truncatula phosphate transporter (MtPT4) that is essential for both the acquisition of phosphate delivered by the AM fungus and is a requirement for the AM symbiosis. The Javot et al. (2007) study used RNAi strategies to downregulate MtPT4, and MtPT4 loss-of-function mutants were identified by TILLING. It was shown that MtPT4 is a low affinity phospate transporter and is a member of a unique clade of phosphate transporters (Pht1, subfamily 1) which are only expressed in the AM symbiosis. The MtPT4 transporter is present in the M. truncatula periarbuscular membrane which forms a continuum with the plasma membrane of the M. truncatula cortical cell (Harrison et al. 2002).
The nematode–plant interaction
As noted above, there is overlap in the signalling pathways between rhizobia and AM symbionts and it is of interest to widen this comparison to include other microbe interactions such as with pathogenic nematodes (Mathesius 2003). The model legumes M. truncatula and L. japonicus can act as hosts for nematodes, which enables comparisons with the plant recognition of rhizobia and AM fungi and the subsequent signalling events (Bird 2004). Root knot nematodes (RKN) induce giant cells in the vascular cylinder where the RKN feed. There is evidence that nematode signalling at the root surface is influenced by mutations in Nod factor receptors (Bird 2004; Weerasinghe et al. 2005).
Plant–aphid interactions
Plant breeders have recognised for some time that annual Medicago species have a range of insect resistances and that there is a range of aphid resistance within M. truncatula cultivars and genotypes (Lake 1989). Development of M. truncatula as a model legume opened up genetic and molecular studies in this area (Klingler et al. 2006; Gao et al. 2008). Different resistance genes exist for spotted alfalfa (Therioaphis trifolii (Monell) f. maculata), pea (Acyrthosiphon pisum Harris) and blue green aphid (Acyrthosiphon kondoi Shinji) (Klingler et al. 2006; Gao et al. 2008). Using M. truncatula, Klingler et al. (2005) identified a single dominant gene which conferred resistance to the bluegreen aphid which is flanked by NBS-LRR resistance gene analogues. Salicylic acid- and ethylene-responsive genes were induced in both resistant and sensitive plants (Gao et al. 2007). However, 10 genes associated with jasmonate signalling were only induced in the aphid resistant line.
Medicago truncatula and pathogenic fungi
Medicago truncatula is also being increasingly used as a model to study pathogen resistance mechanisms (Ellwood et al. 2006b; Tivoli et al. 2006; Foster-Hartnett et al. 2007; Samac and Graham 2007). The value and basis of M. truncatula as a model for nectrotrophic pathogens has been reviewed by Tivoli et al. (2006). The group at the Australian Centre for Necrotrophic Fungal Pathogens is making use of the world’s largest collection of M. truncatula accessions, curated by SARDI at the University of Adelaide Waite Campus. The collection has been shown to be highly diverse, with over 90% of individuals showing discrete genotypes (Ellwood et al. 2006a). Sources of resistance to Phoma medicaginis Malbr. & Roum. have been identified in the SARDI collection (Ellwood et al. 2006b). Legume powdery mildew caused by the biotroph Erysiphe pisi DC. has been investigated by Foster-Hartnett et al. (2007) using M. truncatula genotypes with different levels of resistance. This latter study includes microarray analysis of gene expression in three genotypes with moderate and high resistance, and susceptibility. This microarray analysis joins others on nodulation (Lohar et al. 2006) and AM infection (Hohnjec et al. 2006) and such data being accumulated will be valuable to link to each other and the mutant and single gene molecular studies. M. truncatula is also a useful pathosystem for root rot diseases caused by Aphanomyces euteiches Drechsler (Gaulin et al. 2007), for Phytophthora (Salzer et al. 2000) and for the soilborne bacterial wilt pathogen Ralstonia solanacearum (Smith) Yabuuchi et al. (Vailleau et al. 2007).
Medicago truncatula and plant development
Nodulation
As well as being a structure that houses the rhizobia the legume nodule enables a suitable environment for nitrogenase to conduct symbiotic biological nitrogen fixation (Oldroyd 2007) and provides an interesting model for plant cell differentiation and morphogenesis. The nodule is initiated opposite xylem poles from differentiated cells of the inner cortex (Beveridge et al. 2007) which means that these cells have to dedifferentiate and re-enter the cell cycle. A primordium ultimately forms and a mature nodule is produced. In the case of M. truncatula, the indeterminate nodule forms an apical meristem from which the nodule develops. As with plant development in general the hormones auxin and cytokinin are key players in nodule morphogenesis (Beveridge et al. 2007). Evidence from M. truncatula RNA interference studies against the cytokinin receptor CRE1 has shown that cytokinin acts as a positive regulator of nodulation (Gonzalez-Rizzo et al. 2006). In L. japonicus, a gain of function mutation in the cytokinin receptor Lotus histidine kinase 1 triggers spontaneous nodule formation in the absence of rhizobia (Tirichine et al. 2007). An LHK1 loss of function mutant fails to initiate cortical cell divisions in response to rhizobial signalling, but infection thread formation occurs (Murray et al. 2007). It is argued that cytokinin is necessary and sufficient for dedifferentiation and cell proliferation leading to root nodule formation (Tirichine et al. 2007); so how is auxin involved in nodule development? Auxin is thought to be involved at different stages of nodule formation and is required for differentiation of the vasculature (de Billy et al. 2001). Auxin accumulates at the site of nodule initiation and it has been shown (Pii et al. 2007) that an increased rhizobia auxin synthesis promotes the formation of indeterminate nodules (as in M. truncatula) but not in determinate nodules.
Lateral root formation
Lateral root formation has been extensively studied in Arabidopis (Casimiro et al. 2003), but the use of legumes provides an interesting perspective as lateral root formation has some similarities to nodule morphogenesis (Beveridge et al. 2007). Lateral root primordia originate from pericycle cells and then the lateral root produces a meristem at the apex, as do indeterminate nodules (Beveridge et al. 2007). Auxin can stimulate lateral root formation in Arabidopsis (Hirota et al. 2007), and recent studies in M. truncatula with IAA-overproducing nodules showed increased lateral root formation (Pii et al. 2007). There may be an overlap between the early events in nodulation and lateral root formation (Beveridge et al. 2007); however, the response to cytokinin differs in nodulation and lateral root formation in M. truncatula. In the case of CRE1 RNAi knockdowns where nodulation did not occur there is strong additional evidence for cytokinin control of nodulation (Gonzalez-Rizzo et al. 2006), but in the case of lateral roots MtCRE1 RNAi roots showed enhanced lateral root density. Clearly, though there are conceptual similarities and some specific overlap between meristem ontogeny, the specific type of meristem produced ultimately requires the expression of a different gene set.
Somatic embryogenesis and organogenesis in vitro
Medicago truncatula forms an indeterminate nodule and conceptually it illustrates the ability of plant cells to dedifferentiate, re-enter the cell cycle and differentiate into an organ containing a meristem. In vitro systems in plant biology function in a conceptually similar way and offer the opportunity to investigate cellular differentiation mechanisms and to experimentally modify the system in different ways (e.g. through media additives). In the development of transformation techniques for M. truncatula lines were developed, such as 2HA, with a greatly enhanced somatic embryogenesis compared with wild type. By modifying the hormones it is possible to obtain adventitious roots in both 2HA and wild type. These developmental outcomes in response to hormones and genotype are shown in Fig. 2.
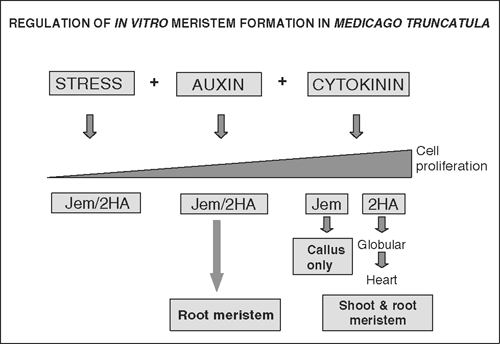
|
Somatic embryogenesis
Somatic embryogenesis, like nodulation, offers fundamental insights into differentiation mechanisms. Somatic embryogenesis is a natural occurring asexual reproductive process in some plants (Garcês et al. 2007). Regeneration by somatic embryogenesis is an important pathway for transformation in many species but as in M. truncatula is often restricted to a specific cultivar, although the reason for this is unknown. The understanding of somatic embryogenesis will also enhance our understanding of apomixis (asexual seed formation, where the genotype is the same as the mother plant) as well as zygotic embryogenesis. In this latter case, an important example is the SERK1 gene, a leucine rich repeat receptor-like kinase, discovered in somatic embryogenesis (Schmidt et al. 1997) and expressed in apomictic (Tucker et al. 2003) and zygotic embryogenesis (Hecht et al. 2001). MtSERK1 is important in somatic embryogenesis and also in in vitro auxin-induced root formation in M. truncatula (Nolan et al. 2003).
An unusual biological aspect of somatic embryogenesis in M. truncatula is that all the very highly regenerable genotypes have been derived after a cycle of tissue culture. These are Jemalong 2HA (Nolan et al. 1989; Rose et al. 1999), R108 (Hoffmann et al. 1997) and M9–10a (Araújo et al. 2004). This suggests that the regeneration process has consistently selected for a somatic cell with a somatic embryogenesis capacity, which is then inherited. As a cycle of tissue culture is always enough to enhance regenerability (Nolan et al. 1989), it suggests that the frequency is too high for a mutation and may be an epigenetic effect.
Somatic embryogenesis in M. truncatula requires a special genotype such as 2HA, auxin plus cytokinin plus the stress of the culture process, and research is in progress to define the signalling pathways involved (Rose and Nolan 2006). Isolated M. truncatula mesophyll protoplasts can regenerate via somatic embryogenesis and cell dedifferentiation and the first cell division cycle can be readily studied in these cells (Sheahan et al. 2005). In both M. truncatula and Arabidopsis massive mitochondrial fusion followed by fission is characteristic and relates to mitochondrial genetics (Sheahan et al. 2005).
Adventitious root formation in vitro
Adventitious root formation can be induced in cultured M. truncatula leaf explants by auxin in both 2HA and wild type (Nolan and Rose 1998; Fig. 2). Although this in vitro response has been known for 50 years in other species (Skoog and Miller 1957), it provides a useful system to study organ differentiation in the M. truncatula context. Histological examination of this system shows that root meristems are initiated de novo from procambial-like cells in the vasculature (Rose et al. 2006; Imin et al. 2007) and auxin-induced root formation is promoted in ethylene transduction mutants such as sickle which is most likely an ein2 mutation. It is of interest to compare in vitro root formation regulation with that of nodules in M. truncatula. With the sickle mutant, there is stimulation of nodule number formation (Prayitno et al. 2006) which suggests that there is some commonality in committing pluripotent cells to a developmental pathway. In this later case ethylene is thought to modulate auxin transport (Prayitno et al. 2006). The value of organogenesis in an in vitro system is similar to that for embryogenesis in that it lends itself to media manipulation and the ready collection of material for high throughput analysis.
Seed development
Given the economic importance of grain legumes, seed development is an important area of investigation in this group of plants. A proteomic study of seed development in M. truncatula (cv. Jemalong line J5) at different stages of seed filling indicated the value of M. truncatula as a model for the analysis of seed filling in legumes (Gallardo et al. 2003). Studies by Djemel et al. (2005) on seed development and composition also concluded that M. truncatula was a suitable model for genomic approaches to seed development in grain legumes. During maturation protein and oil accumulated at fairly constant rates (Djemel et al. 2005), but the major protein groups were shown to accumulate in a specific temporal order (Gallardo et al. 2003). Firnhaber et al. (2005) have conducted microarray studies in flower pods and found more than 700 genes to be developmentally regulated.
Medicago truncatula and stress biology
Medicago truncatula is a valuable species to study how a plant interacts with its environment. It has the capacity to recognise signals from beneficial organisms and to have appropriate developmental responses. It also has to combat disease, pests and environmental stressors by recognising and responding to these signals to enable continuing growth and development. Soil salinity is a significant stressor for crop plants and adaptation of root development has been studied by Merchan et al. (2007) in M. truncatula. An AP2 transcription factor MtZpt2–1 when overexpressed in M. truncatula plants carrying Agrobacterium rhizogenes transformed roots allowed sustained root growth under salt stress conditions. Another interesting feature of the study was that two genes homologous to cytokinin receptors were induced in the salt recovery phase. Hormones are known to be critical in root system growth and architecture and are important in responding to abiotic stress (Malamy 2005). In legumes it is interesting that cytokinin has a key role in nodule formation (Gonzalez-Rizzo et al. 2006) but inhibits root formation in vitro (Nolan and Rose 1998) and cytokinins in M. truncatula are negative regulators of lateral root formation (Gonzalez-Rizzo et al. 2006).
Drought tolerance investigations have also been carried out utilising M. truncatula genes. Zhang et al. (2005) have overexpressed WXP1 a putative AP2 domain-containing transcription factor gene. This gene when overexpressed increased the cuticular wax and enhanced drought tolerance. It is noteworthy that MtZpt2–1 is also an AP2 transcription factor and has a single Ap2 domain which places it in the Dreb sub-family of the AP2/ERF super family. The AP2/ERF transcription family has some particularly interesting features as its members include genes related to abiotic and biotic stress and development (Nakano et al. 2006) which is suggestive of evolutionary forces connecting stress to development. Mantiri et al. (2008) have also recently found that a member of the ERF family of transcription factors is essential for somatic embryogenesis, possibly linking the stress of the culture process to development (Mantiri et al. 2008).
Transcription factors are clearly important regulators of both development and abiotic stress tolerance. In addition to the examples given, of MtZpt2–1 and WXP1 in relation to salt and drought stress, other legume transcription factors have been implicated in abiotic stress tolerance (Udvardi et al. 2007). As Udvardi and coauthors have pointed out (Udvardi et al. 2007), evolution has endowed plants with the ability to ensure their growth and development while fixed in space and subject to environmental extremes. Less than 1% of the more than 2000 transcription factors (TFs) in the model legumes (Medicago and Lotus) have been functionally characterised, so there is much scope to discover new strategies for using genetic means to influence stress tolerance (Udvardi et al. 2007). The Udvardi et al. (2007) legume transcription factor update has a lists of legume TFs that have been genetically characterised and those that have been characterised biochemically and molecularly. Also there is a useful guide to domain shuffling between Medicago TF families in this latter article.
Conclusions and future prospects for research using M. truncatula
From the research discussed it can be seen that M. truncatula has emerged as an important model legume which has facilitated advances in the legume symbioses and opened up new areas of research into biotic stress, plant responses to pests and pathogens and plant development. There is a platform for continued progress in these areas. The completion of sequencing to capture most of the genes and the continued evolution of the bioinformatics is clearly important. As genome science progresses it seems likely that there will be a need to have a complete genome sequence, which given the advances in gene sequencing should be an attainable goal. Arabidopsis research has greatly benefited from the availability of insertional mutants which has made the identification of the function of all genes a realistic proposition. Though advances have been made, really high throughput transformation is still not possible in M. truncatula. There has been a promising recent study in M. sativa (Weeks et al. 2008) on in planta transformation directed at the apical meristem of the seedling, which are cut at the seedling node. Some specific areas where future M. truncatula research could provide further insights into legume and plant biology are highlighted below.
Can symbiosis be engineered?
With the increasing understanding of the signalling pathways involved in nodule and arbuscule signalling (Oldroyd and Downie 2006) and the demonstration that gain-of-function mutations in CCaMK and LHK1 genes can cause spontaneous nodule formation in the absence of rhizobial bacteria (Gleason et al. 2006; Tirichine et al. 2007); the possibility of transferring symbiotic processes into other plant species has again been raised (Oldroyd 2007). Today, this possibility, with current genetic and genomic tools, increasingly looks a more realistic goal.
Small RNAs
There is another level of regulatory control that needs to be considered in understanding the regulation of plant processes. Gene expression can be regulated by RNA-induced gene silencing involving micro-RNAs (miRNAs) or short interfering RNAs (siRNAS) 21–24 nt in length (Jones-Rhoades et al. 2006; Axtell et al. 2007; Brosnan et al. 2007). The siRNAs and miRNAs guide argonaute-like proteins to mediate mRNA degredation, translational repression or transcriptional silencing (Jones-Rhoades et al. 2006; Brosnan et al. 2007). In plants, mRNA silencing can be transmitted from cell to cell and from roots to shoots (Brosnan et al. 2007). Many of the miRNAs regulate developmental processes (Jones-Rhoades et al. 2006). Small RNAs have received minimal attention in legumes.
Connecting plant growth and development to the abiotic and biotic environment
As highlighted in a recent opinion article (Potters et al. 2007), plants have to ultimately grow and develop out of trouble caused by environmental stressors. Legumes have this fascinating ability to have both symbiotic and defence responses leading to some different insights into the control of the diverse signalling pathways that contribute to the life of the legume plant. There are useful comparisons and overlaps in the area of plant–microbe interaction as well as in legume development (Beveridge et al. 2007). The biology of receptors is an area where functional overlaps are providing different perspectives. An example here is the SERK family of receptors (SERK3 is synonomous with BAK1) with roles in development, brassinosteroid reception and innate immunity (Chinchilla et al. 2007). One group of transcription factors that have interesting signalling connections when thinking of functional integration is the AP2/ERF superfamily of transcription factors (Alonso et al. 2003; Nakano et al. 2006). In M. truncatula, this family is involved in nodulation (Middleton et al. 2007), abiotic stress (Zhang et al. 2005) and development (Mantiri et al. 2008). In other species the role of the AP2/ERF superfamily in pathogen defence signalling is well established (Thatcher et al. 2005).
Acknowledgements
Work in my laboratory on Medicago truncatula has been supported by the Wool Research and Development Corporation, the Grains Research and Development Corporation, The University of Newcastle and currently by an ARC Centre of Excellence Grant for Integrative Legume Research (CILR) to the Universities of Queensland, Melbourne and Newcastle, and the Australian National University (Grant CEO348212). I wish to thank my colleagues from the CILR and my research group for the many stimulating discussions on Medicago truncatula biology; and also Lowell Johnson (KSU) and Ian Kaehne and Andrew Lake (formerly of SARDI) for introducing me to annual Medicago.
Alonso JM,
Stepanova AN,
Leisse TJ,
Kim CJ, Chen H , et al.
(2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Amor BB,
Shaw SL,
Oldroyd GED,
Maillet F,
Penmetsa RV,
Cook D,
Long SR,
Dénarié J, Gough C
(2003) The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. The Plant Journal 34, 495–506.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ané J-M,
Kiss GB,
Riely BK,
Penmetsa RV, Oldroyd GED , et al.
(2004) Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303, 1364–1367.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ané J-M,
Zhu H, Frugoli J
(2008) Recent advances in Medicago truncatula genomics. International Journal of Plant Genomics in press ,

Araújo SS,
Duque ASRLA,
Santos DMMF, Fevereiro MPS
(2004) An efficient transformation method to regenerate a high number of transgenic plants using a new embryogenic line of Medicago truncatula cv. Jemalong. Plant Cell, Tissue and Organ Culture 78, 123–131.
| Crossref | GoogleScholarGoogle Scholar |

Aubert G,
Morin J,
Jacquin F,
Loridon K,
Quillet MC,
Petit A,
Rameau C,
Lejeune-Hénaut I,
Huguet T, Burnstin J
(2006) Functional mapping in pea, as an aid to the candidate gene selection and for investigating synteny with the model legume Medicago truncatula. Theoretical and Applied Genetics 112, 1024–1041.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Axtell MJ,
Snyder JA, Bartel DP
(2007) Common functions for diverse small RNAs of land plants. The Plant Cell 19, 1750–1769.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Barker DG,
Bianchi S,
Blondon F,
Datteé Y, Duc G , et al.
(1990) Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Molecular Biology Reporter 8, 40–49.
| Crossref | GoogleScholarGoogle Scholar |

Benaben V,
Duc C,
Lefebvre V, Huguet T
(1995) TE7, an inefficient symbiotic mutant of Medicago truncatula Gaertn cv Jemalong. Plant Physiology 107, 53–62.
| PubMed |

Bennett MD, Leitch IJ
(1995) Nuclear DNA amounts in angiosperms. Annals of Botany 76, 113–176.
| Crossref | GoogleScholarGoogle Scholar |

Bevan MW,
Flavell RB, Chilton MD
(1983) A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304, 184–187.
| Crossref | GoogleScholarGoogle Scholar |

Beveridge CA,
Mathesius U,
Rose RJ, Gresshoff PM
(2007) Common regulatory themes in meristem development and whole plant homeostasis. Current Opinion in Plant Biology 10, 44–51.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bingham ET,
Hurley LV,
Kaatz DM, Saunders JW
(1975) Breeding alfalfa which regenerates from callus tissue in culture. Crop Science 15, 719–721.

Bird DMcK
(2004) Signaling between nematodes and plants. Current Opinion in Plant Biology 7, 372–376.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Boisson-Dernier A,
Chabaud M,
Garcia F,
Becard G,
Rosenberg C, Barker DG
(2001) Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Molecular Plant–Microbe Interactions 14, 695–700.
| Crossref | GoogleScholarGoogle Scholar |

Brewin NJ
(1991) Development of the legume root nodule. Annual Review of Cell Biology 7, 191–226.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brosnan CA,
Mitter N,
Christie M,
Smith NA,
Waterhouse PM, Carroll BJ
(2007) Nuclear gene silencing directs reception of long-distance mRNA silencing in Arabidopsis. Proceedings of the National Academy of Sciences USA 104, 14741–14746.
| Crossref | GoogleScholarGoogle Scholar |

Cannon SB,
Crow JA,
Heuer ML,
Wang X, Cannon EKS , et al.
(2005) Databases and information integration for the Medicago truncatula genome and transcriptome. Plant Physiology 138, 38–46.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cannon SB,
Sterck L,
Rombauts S,
Sato S, Cheung F , et al.
(2006) Legume genome evolution viewed through the Medicago truncatula and Lotus japonicus genomes. Proceedings of the National Academy of Sciences USA 103, 14959–14964.
| Crossref | GoogleScholarGoogle Scholar |

Carlson PS,
Smith HH, Dearing RD
(1972) Parasexual interspecific plant hybridisation. Proceedings of the National Academy of Sciences USA 69, 2292–2294.
| Crossref | GoogleScholarGoogle Scholar |

Casimiro I,
Beeckman T,
Graham N,
Bhalerao R,
Zhang H,
Casero P,
Sandberg G, Bennett M
(2003) Dissecting Arabidopsis lateral root development. Trends in Plant Science 8, 165–171.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chabaud M,
Larsonneau C,
Marmouget C, Huguet T
(1996) Transformation of barrel medic (Medicago truncatula Gaertn.) by Agrobacterium tumefaciens and regeneration via somatic embryogenesis of transgenic plants with the MtENOD12 nodulin promoter fused to the gus reporter gene. Plant Cell Reports 15, 305–310.

Chabaud M,
de Carvalho-Niebel F, Barker DG
(2003) Efficient transformation of Medicago truncatula cv. Jemalong using the hypervirulent Agrobacterium tumefaciens strain AGL1 Plant Cell Reports 22, 46–51.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chinchilla D,
Zipfel C,
Robatzek S,
Kemmerling B,
Nürnberger T,
Jones JDG,
Felix G, Boller T
(2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497–500.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Choi H-K,
Kim D,
Uhm T,
Limpens E, Lim H , et al.
(2004a) A sequence based genetic map of Medicago truncatula and comparison of marker colinearity with M. sativa. Genetics 166, 1463–1502.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Choi H-K,
Mun J-H,
Kim D-J,
Zhu H, Baek J-M , et al.
(2004b) Estimating genome conservation between crop and model legume species. Proceedings of the National Academy of Sciences USA 101, 15289–15294.
| Crossref | GoogleScholarGoogle Scholar |

Clough SJ, Bent AF
(1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cook DR
(1999) Medicago truncatula – a model in the making! Current Opinion in Plant Biology 2, 301–304.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cook DR,
VandenBosch K,
de Bruijn FJ, Huguet T
(1997) Model legumes get the nod. The Plant Cell 9, 275–281.
| Crossref | GoogleScholarGoogle Scholar |

Covitz PA,
Smith LS, Long SR
(1998) Expressed sequence tags from a root-hair-enriched Medicago truncatula cDNA library. Plant Physiology 117, 1325–1332.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Crane C,
Wright E,
Dixon RA, Wang Z-Y
(2006) Transgenic Medicago truncatula plants obtained from Agrobacterium tumefaciens-transformed roots and Agrobacterium rhizogenes – transformed hairy roots. Planta 223, 1344–1354.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Crawford EJ,
Lake AWH, Boyce KG
(1989) Breeding annual Medicago species for semiarid conditions in Southern Australia. Advances in Agronomy 42, 399–437.

Cullimore J, Dénarié J
(2003) How legumes select their sweet talking symbionts. Plant Science 302, 575–578.

de Billy F,
Grosjean C,
May S,
Bennett M, Cullimore JV
(2001) Expression studies on AUXI-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Molecular Plant–Microbe Interactions 14, 267–277.
| Crossref | GoogleScholarGoogle Scholar |

Djemel N,
Guedon D,
Lechevalier A,
Salon C,
Miquel M,
Prosperi JM,
Rochat C, Boutin JP
(2005) Development and composition of the seeds of nine genotypes of the Medicago truncatula species complex. Plant Physiology and Biochemistry 43, 557–566.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ellwood SR,
D’Souza NK,
Kamphuis LG,
Burgess TI,
Nair RM, Oliver RP
(2006a) SSR analysis of the Medicago truncatula SARDI core collection reveals substantial diversity and unusual genotype dispersal throughout the Mediterranean basin. Theoretical and Applied Genetics 112, 977–983.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ellwood SR,
Kamphuis KL, Oliver RP
(2006b) Identification of sources of resistance to Phoma medicaginis isolates in Medicago truncatula SARDI core collection accessions, and multigene differentiation of isolates. Phytopathology 96, 1330–1336.
| Crossref | GoogleScholarGoogle Scholar |

Firnhaber C,
Puhler A, Kuster H
(2005) EST sequencing and time course microarray hybridisations identify more than 700 M. truncatula genes with developmental expression regulation in flowers and pods. Planta 222, 269–283.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Foster-Hartnett D,
Danesh D,
Peñuela S,
Sharopova N,
Endre G,
Vandenbosch KA,
Young ND, Samac DA
(2007) Molecular and cytological responses of Medicago truncatula to Erysiphe pisi. Molecular Plant Pathology 8, 307–319.
| Crossref | GoogleScholarGoogle Scholar |

Fraley RT,
Rogers SB,
Horsch RB,
Sanders PR, Flick JS , et al.
(1983) Expression of bacterial genes in plant cells. Proceedings of the National Academy of Sciences USA 80, 4803–4807.
| Crossref | GoogleScholarGoogle Scholar |

Galibert F,
Finan TM,
Long SR,
Puhler A, Abola P , et al.
(2001) The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293, 668–672.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gallardo K,
Le Signor C,
Vandekerckhove J,
Thompson RD, Burstin J
(2003) Proteomics of Medicago truncatula seed development establishes the time frame of diverse metabolic processes related to reserve accumulation. Plant Physiology 133, 664–682.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gao L-L,
Anderson JP,
Klingler JP,
Nair RM,
Edwards OR, Singh KB
(2007) Involvement of the octadecanoid pathway in bluegreen aphid resistance in Medicago truncatula. Molecular Plant–Microbe Interactions 20, 82–93.
| Crossref | GoogleScholarGoogle Scholar |

Gao L-L,
Klingler JP,
Anderson JP,
Edwards OR, Singh KB
(2008) Characterization of pea aphid resistance in Medicago truncatula. Plant Physiology 146, 996–1009.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Garcês HMP,
Champagne CEM,
Townsley BT,
Park S,
Malhó R,
Pedroso MC,
Harada JJ, Sinha NR
(2007) Evolution of asexual reproduction in leaves of the genus Kalanchoë. Proceedings of the National Academy of Sciences USA 104, 15578–15583.
| Crossref | GoogleScholarGoogle Scholar |

Gaulin E,
Jacquet C,
Bottin A, Dumas B
(2007) Root rot disease of legumes caused by Aphanomyces eutiches. Molecular Plant Pathology 8, 539–548.
| Crossref | GoogleScholarGoogle Scholar |

Gleason C,
Chaudhuri S,
Yang T,
Muñoz A,
Poovaiah BW, Oldroyd GED
(2006) Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 441, 1149–1152.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gonzalez-Rizzo S,
Crespi M, Frugier F
(2006) The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti The Plant Cell 18, 2680–2693.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Harrison MJ,
Dewbre GR, Liu J
(2002) A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. The Plant Cell 14, 2413–2429.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hecht V,
Vielle-Calzada JP,
Hartog MV,
Schmidt EDL,
Boutilier K,
Grossniklaus U, de Vries SC
(2001) The Arabidopsis somatic embryogenesis receptor kinase 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiology 127, 803–816.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Herrera-Estrella LA,
Depicker M,
van Montagu M, Schell J
(1983) Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature 303, 209–213.
| Crossref | GoogleScholarGoogle Scholar |

Hirota A,
Kato K,
Fukaki H,
Aida M, Tasaka M
(2007) The Auxin-regulated AP2/EREBP gene PUCHI is required for morphogenesis in the early lateral root primordium of Arabidopsis. The Plant Cell 19, 2156–2168.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hoffmann B,
Trinh TH,
Leung J,
Kondorosi A, Kondorosi E
(1997) A new Medicago truncatula line with superior in vitro regeneration, transformation, and symbiotic properties isolated through cell culture selection. Molecular Plant–Microbe Interactions 10, 307–315.
| Crossref | GoogleScholarGoogle Scholar |

Hohnjec N,
Henckel K,
Bekel T,
Gouzy J,
Dondrup M,
Goesmann A, Küster H
(2006) Transcriptional snapshots provide insights into the molecular basis of arbuscular mycorrhiza in the model legume Medicago truncatula. Functional Plant Biology 33, 737–748.
| Crossref | GoogleScholarGoogle Scholar |

Imin N,
de Jong F,
Mathesius U,
van Noorden G,
Saeed NA,
Wang X-D,
Rose RJ, Rolfe BG
(2004) Proteome reference maps of Medicago truncatula embryogenic cell cultures generated from single protoplasts. Proteomics 4, 1883–1896.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Imin N,
Nizamidin M,
Daniher D,
Nolan KE,
Rose RJ, Rolfe BG
(2005) Proteomic analysis of somatic embryogenesis in Medicago truncatula. Explant cultures grown under 6-Benzylaminopurine and 1-Naphthaleneacetic acid treatments. Plant Physiology 137, 1250–1260.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Imin N,
Nizamidin M,
Wu T, Rolfe BG
(2007) Factors involved in root formation in Medicago truncatula. Journal of Experimental Botany 58, 439–451.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Javot H,
Penmetsa RV,
Terzaghi N,
Cook DR, Harrison MJ
(2007) A Medicago truncatula transporter indispensable for the arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences USA 104, 1720–1725.
| Crossref | GoogleScholarGoogle Scholar |

Jayasena KW,
Hajimorad MR,
Law EG,
Rehman A-U,
Nolan KE,
Zanker T,
Rose RJ, Randles JW
(2001) Resistance to Alfalfa mosaic virus in transgenic barrel medic lines containing the virus coat protein gene. Australian Journal of Agricultural Research 52, 67–72.
| Crossref | GoogleScholarGoogle Scholar |

Johnson LB,
Stuteville DL,
Higgins RK, Skinner DZ
(1981) Regeneration of alfalfa plants from protoplasts of selected Regen S clones. Plant Science Letters 20, 297–304.
| Crossref | GoogleScholarGoogle Scholar |

Jones-Rhoades MW,
Bartel DP, Bartel B
(2006) MicroRNAs and their regulatory roles in plants. Annual Review of Plant Biology 57, 19–53.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kaló P,
Gleason C,
Edwards A,
Marsh J, Mitra RM , et al.
(2005) Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308, 1786–1789.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Klingler J,
Creasy R,
Gao L,
Nair RM,
Calix AS,
Jacob HS,
Edwards OR, Singh KB
(2005) Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiology 137, 1445–1455.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Klingler JP,
Edwards OR, Singh KB
(2006) Independent action and contrasting phenotypes of resistance genes against spotted alfalfa aphid and bluegreen aphid in Medicago truncatula. New Phytologist 173, 630–640.
| Crossref |

Kulikova O,
Gualtieri G,
Geurts R,
Kim D-J,
Cook D,
Huguet T,
de Jong JH,
Fransz PF, Bisseling T
(2001) Integration of the FISH pachytene and genetic maps of Medicago truncatula. The Plant Journal 27, 49–58.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kulikova O,
Geurts R,
Lamine M,
Kim DJ,
Cook DR,
Leunissen J,
de Jong H,
Roe BA, Bisseling T
(2004) Satellite repeats in the functional centromere and pericentromeric heterochromatin of Medicago truncatula. Chromosoma 113, 276–283.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lake AWH
(1989) Spotted alfalfa aphid survival and reproduction on annual medics with various levels of aphid resistance. Australian Journal of Agricultural Research 40, 117–123.
| Crossref | GoogleScholarGoogle Scholar |

Lévy J,
Bres C,
Geurts R,
Chalhoub B, Kulikova O , et al.
(2004) A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303, 1361–1364.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Limpens E,
Mirabella R,
Fedorova E,
Franken C,
Franssen H,
Bisseling T, Geurts R
(2005) Formation of organelle-like N2-fixing symbiosomes in legume root nodules is controlled by DM12. Proceedings of the National Academy of Sciences USA 102, 10375–10380.
| Crossref | GoogleScholarGoogle Scholar |

Liu J,
Blaylock LA,
Endre G,
Cho J,
Town CD,
VandenBosch KA, Harrison MJ
(2003) Transcript profiling coupled with spatial expression analyses reveals genes involved in distinct developmental stages of an arbuscular mycorrhizal symbiosis. The Plant Cell 15, 2106–2123.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lohar DP,
Sharopova N,
Endre G,
Penuela S,
Samac D,
Town C,
Silverstein KAT, VandenBosch KA
(2006) Transcript analysis of early nodulation events in Medicago truncatula. Plant Physiology 140, 221–234.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Loi A,
Nutt BJ,
McRobb R, Ewing MA
(2000) Potential new alternative annual pasture legumes for Australian Mediterranean farming system. Cahiers Options Méditerranéennes 45, 51–54.

Loi A,
Howieson JG,
Nutt BJ, Carr SJ
(2005) a second generation of annual pasture legumes and their potential for inclusion in Mediterranean-type farming systems. Australian Journal of Experimental Agriculture 45, 289–299.
| Crossref | GoogleScholarGoogle Scholar |

Malamy JE
(2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell & Environment 28, 67–77.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mantiri FR,
Kurdyukov S,
Lohar DP,
Sharopova N,
Saeed NA,
Wang X-D,
VandenBosch KA, Rose RJ
(2008) The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiology 146, 1622–1636.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mathesius U
(2003) Conservation and divergence of signalling pathways between roots and soil microbes – the Rhizobium-legume symbiosis compared to the development of lateral roots, mycorrhizal interactions and nematode induced galls. Plant and Soil 255, 105–119.
| Crossref | GoogleScholarGoogle Scholar |

Mathesius U,
Keijzers G,
Natera SH,
Weinman JJ,
Djordjevic MA, Rolfe BG
(2001) Establishment of a root proteome reference map for the model legume Medicago truncatula using the expressed sequence tag database for peptide mass fingerprinting. Proteomics 1, 1424–1440.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Merchan F,
de Lorenzo L,
Rizzo SG,
Niebel A,
Manyani H,
Frugier F,
Souse C, Crespi M
(2007) Identification of regulatory pathways involved in the reacquisition of root growth after salt stress in Medicago truncatula. The Plant Journal 51, 1–17.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Middleton PH,
Jakab J,
Penmetsa RV,
Starker CG, Doll J , et al.
(2007) An ERF transcription factor in Medicago truncatula that is essential for nod factor signal transduction. The Plant Cell 19, 1221–1234.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Millar AH,
Heazlewood JL,
Kristensen BK,
Braun H-P, Møller IM
(2005) The plant mitochondrial proteome. Trends in Plant Science 10, 36–43.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Murai N,
Sutton DW,
Murray MG,
Slightom JL, Merlo DJ , et al.
(1983) Phaseolin gene from bean is expressed after transfer to sunflower via tumor-inducing plasmid vectors. Science 222, 476–482.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Murray JD,
Karas BJ,
Sato S,
Tabata S,
Amyot L, Szczyglowski K
(2007) A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315, 101–104.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nakano T,
Suzuki K,
Fujimura T, Shinshi H
(2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiology 140, 411–432.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nam Y-W,
Penmetsa RV,
Endre G,
Uribe P,
Kim D, Cook DR
(1999) Construction of a bacterial artificial chromosome library of Medicago truncatula and identification of clones containing ethylene-response genes. Theoretical and Applied Genetics 98, 638–646.
| Crossref | GoogleScholarGoogle Scholar |

Nolan KE, Rose RJ
(1998) Plant regeneration from cultured Medicago truncatula with particular reference to abscisic acid and light treatments. Australian Journal of Botany 46, 151–160.
| Crossref | GoogleScholarGoogle Scholar |

Nolan KE,
Rose RJ, Gorst JE
(1989) Regeneration of Medicago truncatula from tissue culture: increased somatic embryogenesis from regenerated plants. Plant Cell Reports 8, 278–281.
| Crossref | GoogleScholarGoogle Scholar |

Nolan KE,
Irwanto RR, Rose RJ
(2003) Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiology 133, 218–230.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Oldroyd GED
(2007) Nodules and hormones. Science 315, 52–53.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Oldroyd GED, Downie JA
(2006) Nuclear calcium changes at the core of symbiosis signalling. Current Opinion in Plant Biology 9, 351–357.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Parniske M
(2004) Molecular genetics of the arbuscular mycorrhizal symbiosis. Current Opinion in Plant Biology 7, 414–421.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Penmetsa RV, Cook DR
(1997) A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275, 527–530.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Penmetsa RV, Cook DR
(2000) Production and characterization of diverse developmental mutants of Medicago truncatula. Plant Physiology 123, 1387–1397.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pii Y,
Crimi M,
Cremonese G,
Spena A, Pandolfini T
(2007) Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biology 7, 21.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Potters G,
Pasternak TP,
Guisez Y,
Palme KJ, Jansen MAK
(2007) Stress-induced morphogenic responses: growing out of trouble? Trends in Plant Science 12, 98–105.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Prayitno J,
Rolfe BG, Mathesius U
(2006) The ethylene-insensitive sickle mutant of Medicago truncatula shows altered auxin transport regulation during nodulation. Plant Physiology 142, 168–180.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Riely BK,
Ané J-M,
Penmetsa RV, Cook DR
(2004) Genetic and genomic analysis in model legumes bring Nod-factor signalling to center stage. Current Opinion in Plant Biology 7, 408–413.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rose RJ, Nolan KE
(1995) Regeneration of Medicago truncatula from protoplasts isolated from kanamycin-sensitive and kanamycin-resistant plants. Plant Cell Reports 14, 349–354.
| Crossref | GoogleScholarGoogle Scholar |

Rose RJ, Nolan KE
(2006) Genetic regulation of somatic embryogenisis with particular reference to Arabidopsis thaliana and Medicago truncatula. In Vitro Cellular & Developmental Biology - Plant 42, 473–481.

Rose RJ,
Johnson LB, Kemble RJ
(1986) Restriction endonuclease studies on the chloroplast and mitochondrial DNAs of alfalfa (Medicago sativa L.) protoclones. Plant Molecular Biology 6, 331–338.
| Crossref | GoogleScholarGoogle Scholar |

Rose RJ,
Nolan KE, Bicego L
(1999) The development of the highly regenerable seed line Jemalong 2HA for transformation of Medicago truncatula – implications for regenerability via somatic embryogenesis. Journal of Plant Physiology 155, 788–791.

Rose RJ,
Wang X-D,
Nolan KE, Rolfe BG
(2006) Root meristems in Medicago truncatula tissue culture arise from vascular-derived procambial-like cells in a process regulated by ethylene. Journal of Experimental Botany 57, 2227–2235.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sagan M,
Morandi D,
Tarenghi E, Duc G
(1995) Selection of nodulation and mycorrhizal mutants in the model plant Medicago truncatula (Gaertn.) after γ-ray mutagenesis. Plant Science 111, 63–71.
| Crossref | GoogleScholarGoogle Scholar |

Salzer P,
Bonanomi A,
Beyer K,
Vögeli-Lange R,
Aeschbacher RA,
Lange J,
Wiemken A,
Kim D,
Cook DR, Boller T
(2000) Differential expression of eight chitinase genes during mycorrhiza formation, nodulation and pathogen infection. Molecular Plant–Microbe Interactions 13, 763–777.
| Crossref | GoogleScholarGoogle Scholar |

Samac DA, Graham MA
(2007) Recent advances in legume-microbe interactions: recognition, defense resonse, and symbiosis from a genomic perspective. Plant Physiology 144, 582–587.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Saunders J, Bingham ET
(1972) Production of alfalfa plants from callus tissue. Crop Science 12, 804–808.

Schmidt EDL,
Guzzo F,
Toonen MAJ, de Vries SC
(1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124, 2049–2062.
| PubMed |

Schnabel E,
Journet E-P,
de Carvalho-Niebel F,
Duc G, Frugoli J
(2005) The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Molecular Biology 58, 809–822.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Shaver JM,
Oldenburg DJ, Bendich AJ
(2008) The structure of chloroplast DNA molecules and the effect of light on the amount of chloroplast DNA during development in Medicago truncatula. Plant Physiology 146, 1064–1074.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sheahan MB,
McCurdy DW, Rose RJ
(2005) Mitochondria as a connected population: ensuring continuity of the mitochondrial genome during plant cell dedifferentiation through massive mitochondrial fusion. The Plant Journal 44, 744–755.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Skoog F, Miller CO
(1957) Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Symposia of the Society for Experimental Biology 11, 118–131.

Smit P,
Raedts J,
Portyanko V,
Debellé F,
Gough C,
Bisseling T, Geurts R
(2005) NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science 308, 1789–1791.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Somerville CR, Ogren WL
(1979) A phosphoglycolate phosphatase-deficient mutant of Arabidopsis. Nature 280, 833–836.
| Crossref | GoogleScholarGoogle Scholar |

Stacey G,
Libault M,
Brechenmacher L,
Wan J, May D
(2006) Genetics and functional genomics of legume nodulation. Current Opinion in Plant Biology 9, 110–121.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tesfaye M,
Silverstein KAT,
Bucciarelli B,
Samac DA, Vance CP
(2006) The Affymetrix Medicago GeneChip array is applicable for transcript analysis of alfalfa (Medicago sativa) Functional Plant Biology 33, 783–788.
| Crossref | GoogleScholarGoogle Scholar |

Thatcher LF,
Anderson JP, Singh KB
(2005) Plant defence response: what have we learnt from Arabidopsis? Functional Plant Biology 32, 1–19.
| Crossref | GoogleScholarGoogle Scholar |

The Genome Initiative
(2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Thomas MR,
Rose RJ, Nolan KE
(1992) Genetic transformation of Medicago truncatula using Agrobacterium with genetically modified Ri and disarmed Ti plasmids. Plant Cell Reports 11, 113–117.
| Crossref | GoogleScholarGoogle Scholar |

Thoquet P,
Ghérardi M,
Journet E-P,
Kereszt A,
Ané J-M,
Prosperi J-M, Huguet T
(2002) The molecular genetic linkage map of the model legume Medicago truncatula: an essential tool for comparative legume genomics and the isolation of agronomically important genes. BMC Plant Biology 2, 1.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tian D, Rose RJ
(1999) Asymmetric somatic hybridisation between the annual legumes Medicago truncatula and Medicago scutellata. Plant Cell Reports 18, 989–996.
| Crossref | GoogleScholarGoogle Scholar |

Tirichine L,
Imaizumi-Anraku H,
Yoshida S,
Murakami Y, Madsen LH , et al.
(2006) Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441, 1153–1156.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tirichine L,
Sandal N,
Madsen LH,
Radutoiu S,
Albreksten AS,
Sato S,
Asamizu E,
Tabata S, Stougaard J
(2007) A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315, 104–107.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tivoli B,
Baranger A,
Sivasithamparam K, Barbetti MJ
(2006) Annual Medicago: from a model crop challenged by a spectrum of necrotrophic pathogens to a model plant to explore the nature of disease resistance. Annals of Botany 98, 1117–1128.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Trieu AT, Harrison MJ
(1996) Rapid transformation of Medicago truncatula regeneration via shoot organogenesis. Plant Cell Reports 16, 6–11.
| Crossref | GoogleScholarGoogle Scholar |

Tucker MR,
Araujo A-CG,
Paech NA,
Hecht V,
Schmidt EDL,
Rossell J-B,
de Vries SC, Koltunow AMG
(2003) Sexual and apomictic reproduction in Hieracium subgenus Pilosella are closely interrelated developmental pathways. The Plant Cell 15, 1524–1537.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Udvardi MK, Scheible W-R
(2005) GRAS genes and the symbiotic green revolution. Science 308, 1749–1750.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Udvardi MK,
Kakar K,
Wandrey M,
Montanari O, Murray J , et al.
(2007) Legume transcription factors: global regulators of plant development and response to the environment. Plant Physiology 144, 538–549.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vailleau F,
Sartorel E,
Jardinaud MF,
Chardon F,
Genin S,
Huguet T,
Gentzbittel L, Petitprez M
(2007) Characterization of the interaction between the bacterial wilt pathogen Ralstonia solanacearum and the model legume plant Medicago truncatula. Molecular Plant–Microbe Interactions 20, 159–167.
| Crossref | GoogleScholarGoogle Scholar |

Wang JH,
Rose RJ, Donaldson BI
(1996) Agrobacterium-mediated transformation and expression of foreign genes in Medicago truncatula. Australian Journal of Plant Physiology 23, 265–270.

Wang H,
Chen J,
Wen J,
Tadege M,
Li G,
Liu Y,
Mysore KM,
Ratet P, Chen R
(2008) Control of compound leaf development by FLORICAULA/LEAFY ortholog SINGLE LEAFLET1 in Medicago truncatula. Plant Physiology 146, 1759–1772.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Watson BS,
Asirvatham VS,
Wang L, Sumner LW
(2003) Mapping the proteome of barrel medic (Medicago truncatula). Plant Physiology 131, 1104–1123.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Weeks JT,
Ye J, Rommens CM
(2008) Development of an in planta method for transformation of alfalfa (Medicago sativa). Transgenic Research in press ,
| PubMed |

Weerasinghe RR,
Bird DM, Allen NS
(2005) Root-knot nematodes and bacterial Nod factors elicit common signal transduction events in Lotus japonicus. Proceedings of the National Academy of Sciences USA 102, 3147–3152.
| Crossref | GoogleScholarGoogle Scholar |

Young ND,
Mudge J, Ellis THN
(2003) Legume genomes: more than peas in a pod. Current Opinion in Plant Biology 6, 199–204.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Young ND,
Cannon SB,
Sato S,
Kim D,
Cook DR,
Town CD,
Roe BA, Tabata S
(2005) Sequencing the genespaces of Medicago truncatula and Lotus japonicus. Plant Physiology 137, 1174–1181.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhang J-Y,
Broeckling CD,
Blancaflor EB,
Sledge MK, Sumner LW
(2005) Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). The Plant Journal 42, 689–707.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhou X,
Chandrasekharan MB, Hall TC
(2004) High rooting frequency and functional analysis of GUS and GFP expression in transgenic Medicago truncatula A17. New Phytologist 162, 813–822.
| Crossref | GoogleScholarGoogle Scholar |



